概要
The EasySep™ Mouse CD117 (cKIT) Positive Selection Kit is designed to isolate CD117+ cells from single-cell suspensions of bone marrow by positive selection. The cocktail contains a combination of PE-labeled antibodies that is directed against CD117, which are then recognized by antibody complexes that are directed against PE and dextran. Labeled cells are bound to magnetic particles and separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse CD117 (cKIT) Positive Selection Kit | 18757 | All | English |
| Product Information Sheet | RoboSep™ Mouse CD117 (cKIT) Positive Selection Kit with Filter Tips | 18757RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse CD117 (cKIT) Positive Selection Kit | 18757 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse CD117 (cKIT) Positive Selection Kit | 18757 | All | English |
| Safety Data Sheet | RoboSep™ Mouse CD117 (cKIT) Positive Selection Kit with Filter Tips | 18757RF | All | English |
数据及文献
Data
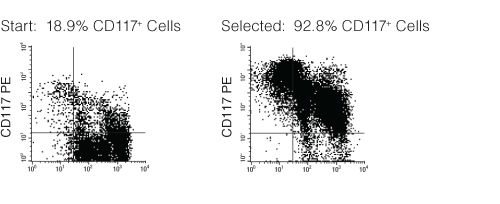
Figure 1. FACS Profile Results with EasySep™ Mouse CD117 (c-KIT) Positive Selection Kit
Starting with mouse bone marrow, the CD117+ cell content of the selected cells typically ranges from 88 - 95%.
Publications (8)
Cell reports 2020 oct
TGF$\beta$R-SMAD3 Signaling Induces Resistance to PARP Inhibitors in the Bone Marrow Microenvironment.
Abstract
Abstract
Synthetic lethality triggered by PARP inhibitor (PARPi) yields promising therapeutic results. Unfortunately, tumor cells acquire PARPi resistance, which is usually associated with the restoration of homologous recombination, loss of PARP1 expression, and/or loss of DNA double-strand break (DSB) end resection regulation. Here, we identify a constitutive mechanism of resistance to PARPi. We report that the bone marrow microenvironment (BMM) facilitates DSB repair activity in leukemia cells to protect them against PARPi-mediated synthetic lethality. This effect depends on the hypoxia-induced overexpression of transforming growth factor beta receptor (TGF$\beta$R) kinase on malignant cells, which is activated by bone marrow stromal cells-derived transforming growth factor beta 1 (TGF-$\beta$1). Genetic and/or pharmacological targeting of the TGF-$\beta$1-TGF$\beta$R kinase axis results in the restoration of the sensitivity of malignant cells to PARPi in BMM and prolongs the survival of leukemia-bearing mice. Our finding may lead to the therapeutic application of the TGF$\beta$R inhibitor in patients receiving PARPis.
Frontiers in immunology 2018
Hematopoietic Stem/Progenitor Cell Dependent Participation of Innate Lymphoid Cells in Low-Intensity Sterile Inflammation.
Abstract
Abstract
Hematopoietic stem/progenitor cells (HSPC) are characterized by their unique capacities of self-renewal and multi-differentiation potential. This second property makes them able to adapt their differentiation profile depending on the local environment they reach. Taking advantage of an animal model of peritonitis, induced by injection of the TLR-2 ligand, zymosan, we sought to study the relationship between bone marrow-derived hematopoietic stem/progenitor cells (BM-HSPCs) and innate lymphoid cells (ILCs) regarding their emergence and differentiation at the site of inflammation. Our results demonstrate that the strength of the inflammatory signals affects the capacity of BM-derived HSPCs to migrate and give rise in situ to ILCs. Both low- and high-dose of zymosan injections trigger the appearance of mature ILCs in the peritoneal cavity where the inflammation occurs. Herein, we show that only in low-dose injected mice, the recovered ILCs are dependent on an in situ differentiation of BM-derived HSPCs and/or ILC2 precursors (ILC2P) wherein high-dose, the stronger inflammatory environment seems to be able to induce the emergence of ILCs independently of BM-derived HSPCs. We suggest that a relationship between HSPCs and ILCs seems to be affected by the strength of the inflammatory stimuli opening new perspectives in the manipulation of these early hematopoietic cells.
Nature communications 2016
Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells.
Abstract
Abstract
The influence of signals perceived by immature B cells during their development in bone marrow on their subsequent functions as mature cells are poorly defined. Here, we show that bone marrow cells transiently stimulated in vivo or in vitro through the Toll-like receptor 9 generate proB cells (CpG-proBs) that interrupt experimental autoimmune encephalomyelitis (EAE) when transferred at the onset of clinical symptoms. Protection requires differentiation of CpG-proBs into mature B cells that home to reactive lymph nodes, where they trap T cells by releasing the CCR7 ligand, CCL19, and to inflamed central nervous system, where they locally limit immunopathogenesis through interleukin-10 production, thereby cooperatively inhibiting ongoing EAE. These data demonstrate that a transient inflammation at the environment, where proB cells develop, is sufficient to confer regulatory functions onto their mature B-cell progeny. In addition, these properties of CpG-proBs open interesting perspectives for cell therapy of autoimmune diseases.
The European Journal of Immunology 2011
Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function
Abstract
Abstract
Mast cells (MCs) play an important role in the regulation of protective adaptive immune responses against pathogens. However, it is still unclear whether MCs promote such host defense responses via direct effects on T cells or rather by modifying the functions of antigen-presenting cells. To identify the underlying mechanisms of the immunoregulatory capacity of MCs, we investigated the impact of MCs on dendritic cell (DC) maturation and function. We found that murine peritoneal MCs underwent direct crosstalk with immature DCs that induced DC maturation as evidenced by enhanced expression of costimulatory molecules. Furthermore, the MC/DC interaction resulted in the release of the T-cell modulating cytokines IFN-γ, IL-2, IL-6 and TGF-β into coculture supernatants and increased the IL-12p70, IFN-γ, IL-6 and TGF-β secretion of LPS-matured DCs. Such MC-primed" DCs subsequently induced efficient CD4+ T-cell proliferation. Surprisingly�
Cancer research 2010 DEC
Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes.
Abstract
Abstract
Chimeric oncoproteins resulting from fusion of MLL to a wide variety of partnering proteins cause biologically distinctive and clinically aggressive acute leukemias. However, the mechanism of MLL-mediated leukemic transformation is not fully understood. Dot1, the only known histone H3 lysine 79 (H3K79) methyltransferase, has been shown to interact with multiple MLL fusion partners including AF9, ENL, AF10, and AF17. In this study, we utilize a conditional Dot1l deletion model to investigate the role of Dot1 in hematopoietic progenitor cell immortalization by MLL fusion proteins. Western blot and mass spectrometry show that Dot1-deficient cells are depleted of the global H3K79 methylation mark. We find that loss of Dot1 activity attenuates cell viability and colony formation potential of cells immortalized by MLL oncoproteins but not by the leukemic oncoprotein E2a-Pbx1. Although this effect is most pronounced for MLL-AF9, we find that Dot1 contributes to the viability of cells immortalized by other MLL oncoproteins that are not known to directly recruit Dot1. Cells immortalized by MLL fusions also show increased apoptosis, suggesting the involvement of Dot1 in survival pathways. In summary, our data point to a pivotal requirement for Dot1 in MLL fusion protein-mediated leukemogenesis and implicate Dot1 as a potential therapeutic target.
Stem cells (Dayton, Ohio) 2009 AUG
c-Kit function is necessary for in vitro myogenic differentiation of bone marrow hematopoietic cells.
Abstract
Abstract
In recent years, the differentiation of bone marrow cells (BMCs) into myocytes has been extensively investigated, but the findings remain inconclusive. The purpose of this study was to determine the conditions necessary to induce myogenic differentiation in short-term cultures of adult BMCs, and to identify the BMC subpopulation responsible for this phenomenon. We report that high-density cultures of murine hematopoietic BMCs gave rise to spontaneous beating cell clusters in the presence of vascular endothelial and fibroblast growth factors. These clusters originated from c-kit(pos) cells. The formation of the clusters could be completely blocked by adding a c-kit/tyrosine kinase inhibitor, Gleevec (imatinib mesylate; Novartis International, Basel, Switzerland, http://www.novartis.com), to the culture. Cluster formation was also blunted in BMCs from c-kit-deficient (Kit(W)/Kit(W-v)) mice. Clustered cells expressed cardiomyocyte-specific transcription factor genes Gata-4 and Nkx2.5, sarcomeric proteins beta-MHC and MLC-2v, and ANF and connexin-43. Immunostaining revealed alpha-sarcomeric actinin expression in more than 90% of clustered cells. Under electron microscopy, the clustered cells exhibited a sarcomeric myofiber arrangement and z-bands. This study defines the microenvironment required to achieve a reproducible in vitro model of beating, myogenic cell clusters. This model could be used to examine the mechanisms responsible for the postnatal myogenic differentiation of BMCs. Our results identify c-kit(pos) bone marrow hematopoietic cells as the source of the myogenic clusters.

 网站首页
网站首页