概要
A flow cytometer equipped with a 488 nm laser and a 635 nm laser for excitation and appropriate filters for detecting APC, PE, PE-Cyanine5 and 7-AAD fluorescence is required for use.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | ALDHbr Assay Kit | 01711 | All | English |
| Product Information Sheet 2 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 1 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 2 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 3 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 4 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 5 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 6 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 7 | ALDHbr Assay Kit | 01711 | All | English |
| Safety Data Sheet 8 | ALDHbr Assay Kit | 01711 | All | English |
数据及文献
Data

Figure 1. General ALDHbr Assay Procedure
For a detailed protocol please refer to the ALDHbr Assay Kit Product Information Sheet (PIS)
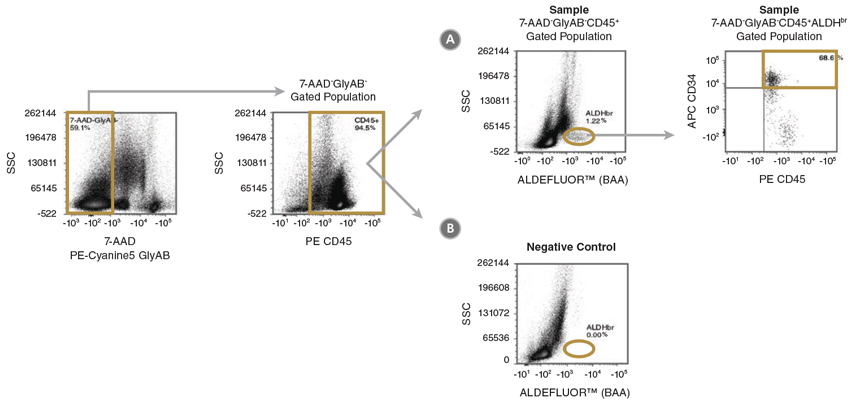
Figure 2. Gating Strategy to Identify 7-AAD-GlyAB-CD45+ALDHbrCD34+ Cells
CB mononuclear cells (106 cells/mL) were stained with the ALDHbr Assay Kit and analyzed on a flow cytometer equipped with 488 nm and 625 nm lasers for detecting ALDEFLUOR™ (BAA), 7-AAD, APC, PE and PE-Cyanine5 fluorescence. The gating strategy for identifying (A) 7-AAD-GlyAB-CD45+ALDHbrCD34+ cells is shown using a fully stained sample (Sample) as described above. Staining of a (B) DEAB negative-control sample (Negative Control) is used to verify the placement of the ALDHbr gate. Gold lines depict the gates used at each stage to identify the desired cell types. Note: The gating of ALDHbr cells is greatly simplified if red blood cells (RBCs) are first removed from CB samples, prior to staining with ALDEFLUOR™. The ErythroClear™ Red Blood Cell Depletion Kit (Catalog #01739) is designed for the depletion of RBCs from up to 16 small volume CB samples.
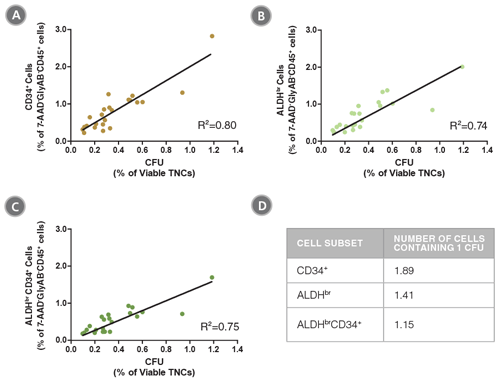
Figure 3. The Frequencies of CD34+, ALDHbr and ALDHbrCD34+ Cells in CB Samples are Correlated with Colony Numbers as Measured in CFU Assays
CB mononuclear cells (n=23) were split into two parts. Half of each sample was stained with the ALDHbr Assay Kit and analyzed, as described in Figure 1. The percentages of (A) CD34+, (B) ALDHbr and (C) ALDHbrCD34+ cells in the 7-AAD-GlyAB-CD45+ population in each sample was determined. The other half of each sample was plated in a CFU assay using MethoCult™ Classic H4434 medium (Catalog #04434) and colonies were counted 14 days later. The frequency of CFUs was determined as a percentage of viable total nucleated cells (TNCs) for each sample. The table in (D) shows the number of cells from each subpopulation that contains 1 CFU. The data show that the frequency of ALDHbrCD34+ cells is highly correlated with the frequency of functional progenitor cells (R2: coefficient of determination).
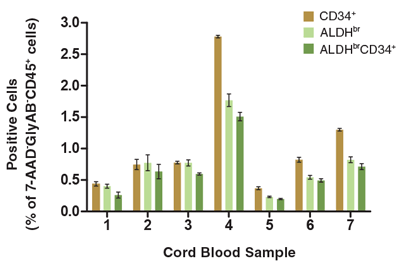
Figure 4. The Percentage of Viable CD45+ Cells that Express CD34+ and/or ALDHbr Vary Among Different CB Samples
CB mononuclear cells (106 cells/mL) were stained and analyzed as described (Figure 1). Shown are the percentages of cells in the 7-AAD-GlyAB-CD45+ gated population that are CD34+, ALDHbr and ALDHbrCD34+. Each column represents the mean ± S.D. of three replicate assays for each CB sample (n=7).
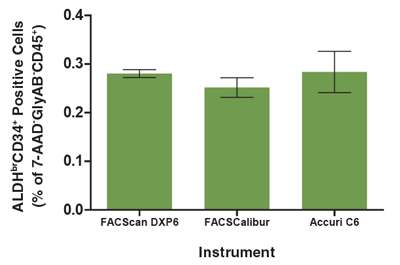
Figure 5. The Analysis of the ALDHbrCD34+ Cell Subset is Consistent Across Different Flow Cytometers
A single CB mononuclear cell sample (106 cells/mL) was split into 3 parts, each of which was separately stained with the ALDHbr Assay Kit (see Figure 1 for protocol) and separately analyzed on three different flow cytometry instruments; a FACScan DXP6, FACSCalibur and Accuri C6. All instruments were equipped with 488 nm and 635 nm lasers for detecting ALDEFLUOR™ (BAA), 7-AAD, APC, PE and PE-Cyanine5 fluorescence. The frequency of ALDHbrCD34+ cells in each sample was determined as a percentage of the 7-AAD-GlyAB-CD45+ cell population measured by each instrument. There was no significant difference in the number of ALDHbrCD34+ cells measured by these flow cytometers (p > 0.05). Each column represents the mean ± S.D. for three replicates of the analyzed CB sample evaluated on each flow cytometer.

 网站首页
网站首页