概要
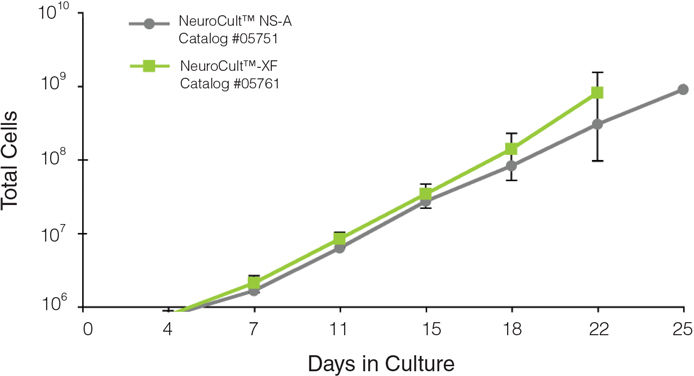
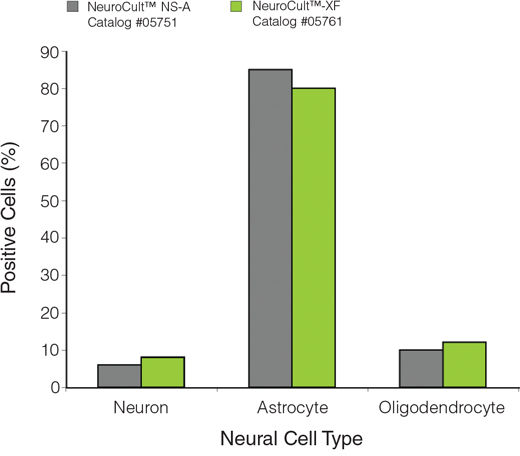
NeuroCult™-XF Proliferation Medium is a standardized, xeno-free, serum-free medium for the culture of human neural stem and progenitor cells from normal tissues or tumor samples, in the neurosphere or adherent monolayer system. When supplemented with appropriate cytokines, NeuroCult™-XF Proliferation Medium is optimized to maintain human neural stem cells in culture for extended periods of time without the loss of their self-renewal, proliferation or differentiation potential.
NOTE: Addition of Carrier-Free rh EGF (Catalog #78006) and rh bFGF (Catalog #78003) is required. Heparin (Catalog #07980) can also be added, however, the heparin solution contains non-human animal-derived components and can be omitted if a complete xeno-free system is desired.
数据及文献
Publications (3)
Bulletin of experimental biology and medicine 2015 AUG
The Use of Autologous Mesenchymal Stem Cells for Cell Therapy of Patients with Amyotrophic Lateral Sclerosis in Belarus.
Rushkevich YN et al.
Abstract
We studied a new method of treatment of amyotrophic lateral sclerosis with autologous mesenchymal stem cells. Autologous mesenchymal stem cells were injected intravenously (intact cells) or via lumbar puncture (cells committed to neuronal differentiation). Evaluation of the results of cell therapy after 12-month follow-up revealed slowing down of the disease progression in 10 patients in comparison with the control group consisting of 15 patients. The cell therapy was safe for the patients.
Annals of translational medicine 2014 OCT
Chemically defined serum-free and xeno-free media for multiple cell lineages.
Usta S et al.
Abstract
Cell culture is one of the most common methods used to recapitulate a human disease environment in a laboratory setting. Cell culture techniques are used to grow and maintain cells of various types including those derived from primary tissues, such as stem cells and cancer tumors. However, a major confounding factor with cell culture is the use of serum and animal (xeno) products in the media. The addition of animal products introduces batch and lot variations that lead to experimental variability, confounds studies with therapeutic outcomes for cultured cells, and represents a major cost associated with cell culture. Here we report a commercially available serum-free, albumin-free, and xeno free (XF) media (Neuro-Pure(TM)) that is more cost-effective than other commercial medias. Neuro-Pure was used to maintain and differentiate various cells of neuronal lineages, fibroblasts, as well as specific cancer cell lines; without the use of contaminants such serum, albumin, and animal products. Neuro-Pure allows for a controlled and reproducible cell culture environment that is applicable to translational medicine and general tissue culture.
Proceedings of the National Academy of Sciences 2013 APR
Hypoxia induces a phase transition within a kinase signaling network in cancer cells
Wei W et al.
Abstract
Hypoxia is a near-universal feature of cancer, promoting glycolysis, cellular proliferation, and angiogenesis. The molecular mechanisms of hypoxic signaling have been intensively studied, but the impact of changes in oxygen partial pressure (pO2) on the state of signaling networks is less clear. In a glioblastoma multiforme (GBM) cancer cell model, we examined the response of signaling networks to targeted pathway inhibition between 21% and 1% pO2. We used a microchip technology that facilitates quantification of a panel of functional proteins from statistical numbers of single cells. We find that near 1.5% pO2, the signaling network associated with mammalian target of rapamycin (mTOR) complex 1 (mTORC1)--a critical component of hypoxic signaling and a compelling cancer drug target--is deregulated in a manner such that it will be unresponsive to mTOR kinase inhibitors near 1.5% pO2, but will respond at higher or lower pO2 values. These predictions were validated through experiments on bulk GBM cell line cultures and on neurosphere cultures of a human-origin GBM xenograft tumor. We attempt to understand this behavior through the use of a quantitative version of Le Chatelier's principle, as well as through a steady-state kinetic model of protein interactions, both of which indicate that hypoxia can influence mTORC1 signaling as a switch. The Le Chatelier approach also indicates that this switch may be thought of as a type of phase transition. Our analysis indicates that certain biologically complex cell behaviors may be understood using fundamental, thermodynamics-motivated principles.
View All Publications

 网站首页
网站首页