概要
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MyoCult™-SF Expansion 10X Supplement (Human) | 05982 | All | English |
| Product Information Sheet | MyoCult™-SF Attachment Substrate | 05983 | All | English |
| Product Information Sheet | MyoCult™-SF Expansion Supplement Kit (Human) | 05980 | All | English |
| Safety Data Sheet 1 | MyoCult™-SF Expansion Supplement Kit (Human) | 05980 | All | English |
| Safety Data Sheet 2 | MyoCult™-SF Expansion Supplement Kit (Human) | 05980 | All | English |
数据及文献
Data
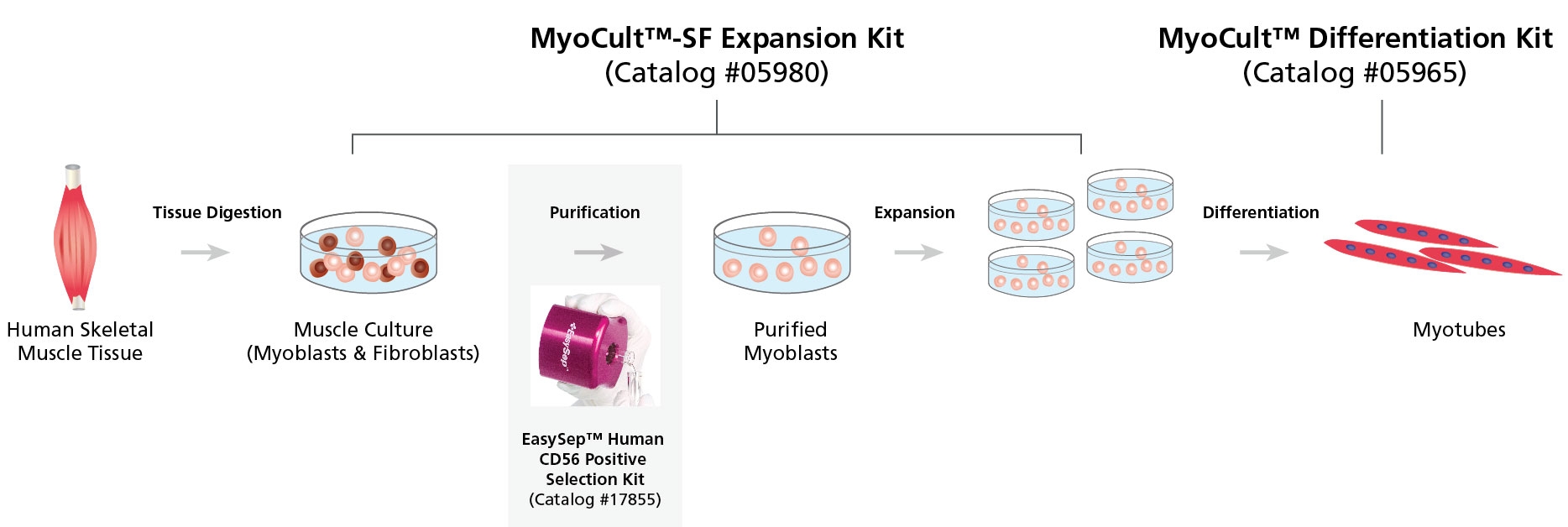
Figure 1. Workflow for the Derivation, Expansion and Differentiation of Human Myogenic Progenitor Cells
Human skeletal muscle tissue is digested into a single cell suspension and plated into MyoCult™-SF Expansion Medium. Myoblasts are then enriched by cell sorting or using a selection kit such as the EasySep™ Human CD56 Positive Selection Kit (Catalog #17855). Purified myoblasts are then culture-expanded or differentiated into myotubes for further studies. Myoblasts can be derived and expanded, using the MyoCult™-SF Expansion Kit (Catalog #05980) and then differentiated using the MyoCult™ Differentiation Kit (Catalog #05965).
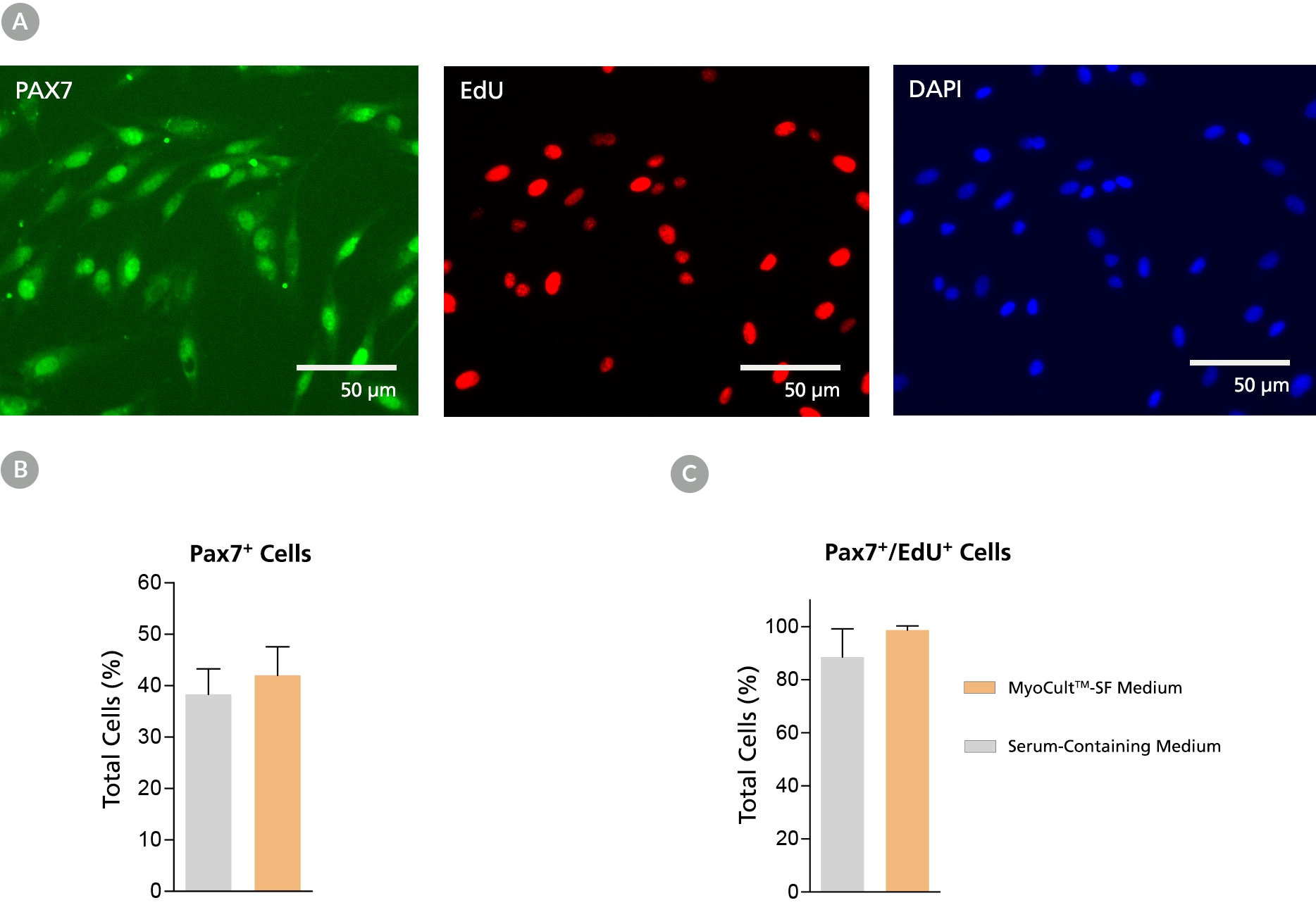
Figure 2. Derivation of PAX7+ Myogenic Progenitor Cells in MyoCult™-SF Expansion Medium from Human Skeletal Muscle Tissue
Myogenic progenitor cells were derived from human skeletal muscle tissue and culture-expanded using the MyoCult™-SF Expansion Kit or a serum-containing medium. (A) Following 6 days of expansion, myoblasts were fixed and immunostained for PAX7 (green), a thymine analogue (EdU; red) and nuclei (DAPI; blue). Total percentage of cells expressing (B) PAX7 or (C) PAX7 and EdU were quantified (n = 4). Data was generated and used with permission by Dr. Penny M. Gilbert’s Lab, Department of Biochemistry, University of Toronto.
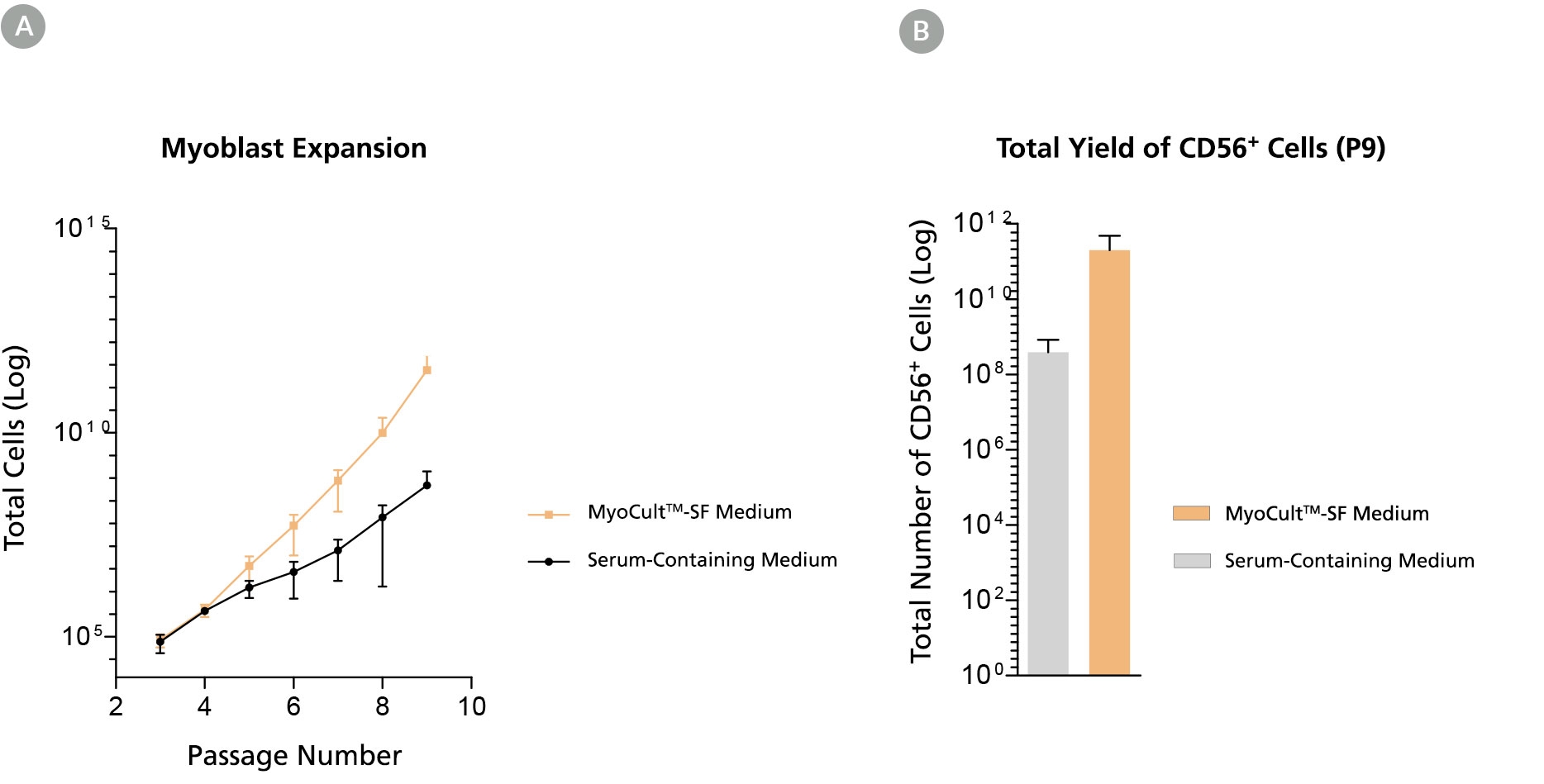
Figure 3. Long-Term Expansion of Myoblasts were Observed When Using MyoCult™-SF Expansion Kit
Myoblasts were culture-expanded in MyoCult™-SF Expansion Medium or in serum-containing medium. (A) Myoblasts culture-expanded in MyoCult™-SF Expansion Medium displayed superior expansion rate than when cells were expanded in serum-containing medium (n=3). (B) Expansion of myoblasts in MyoCult™-SF Expansion Medium generates a greater yield of CD56+ cells after 9 passages compared to myoblasts cultured in serum-containing medium (P9; n = 2). Data was generated and used with permission by Dr. Penny M. Gilbert’s Lab, Department of Biochemistry, University of Toronto.
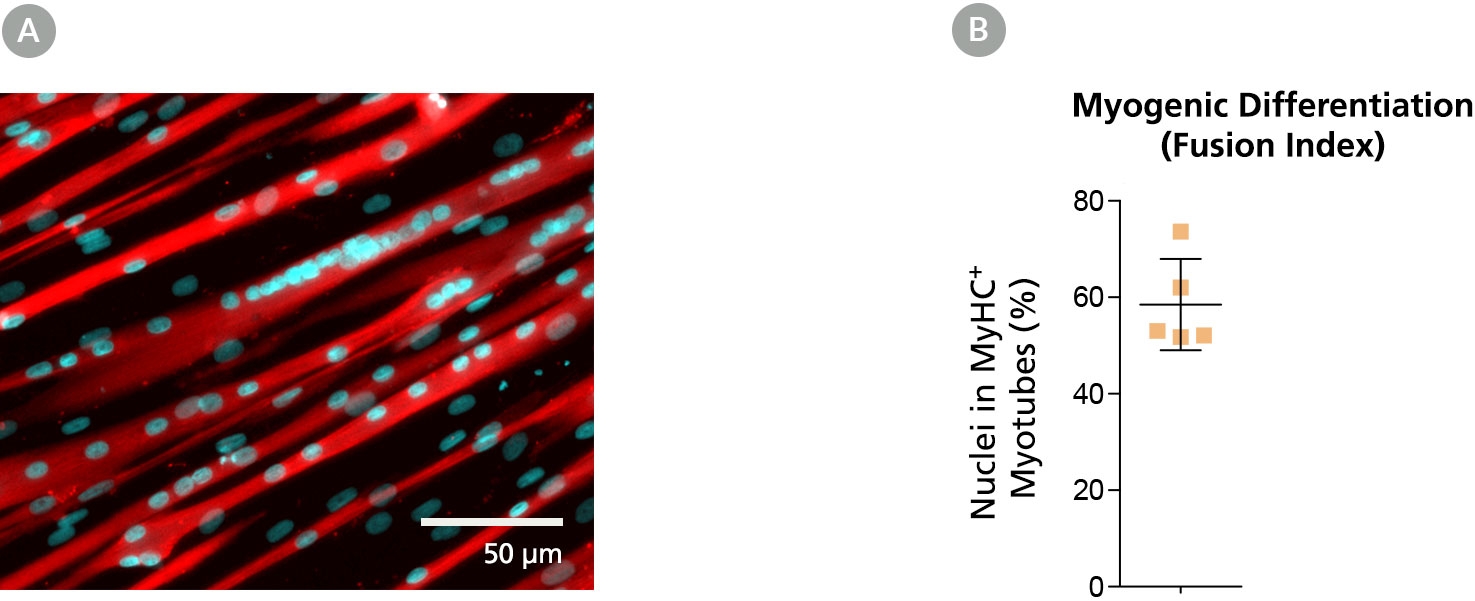
Figure 4. Myoblasts Culture-Expanded in MyoCult™-SF Expansion Medium Maintain Differentiation and Transplantation Potential in Mice
Myoblasts derived and expanded in MyoCult™-SF Expansion Medium were further differentiated into myotubes using the MyoCult™ Differentiation Kit (Catalog #05965) at passage 5. (A) Myotubes differentiated from myoblasts were immunostained for myosin heavy chain (MyHC; red) and nuclei (DAPI; blue). (B) Fusion index displaying approximately 60% of total nuclei were found within myotubes expressing MyHC. Each donor is indicated by a yellow square. Data from 5 independent donors.
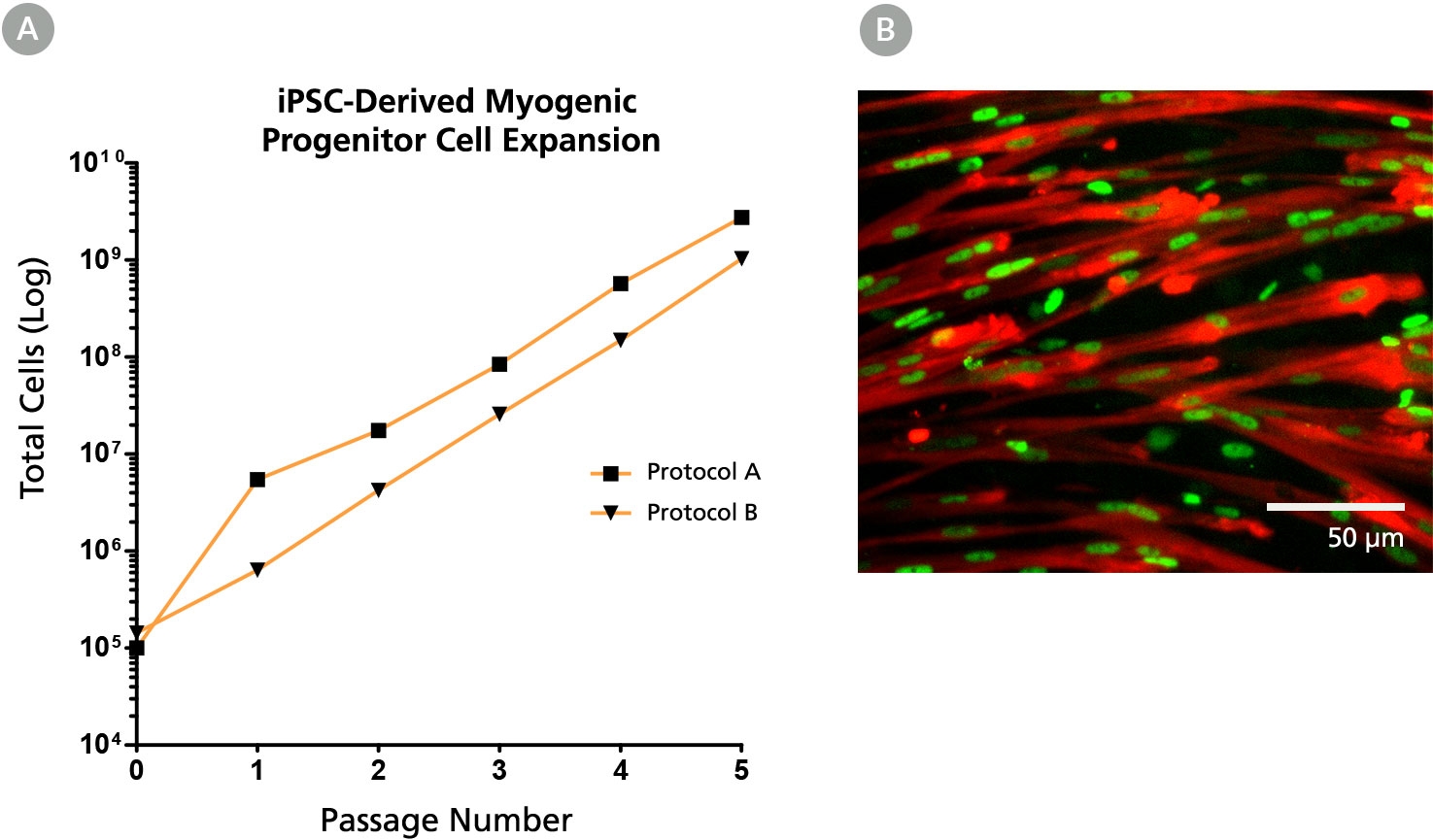
Figure 5. The MyoCult™-SF Expansion Medium Supports Expansion of iPSC-Derived Myogenic Progenitor Cells
(A) iPSC-derived myogenic progenitor cells displayed long-term expansion when using the MyoCult™ Expansion Kit. iPSC-derived myogenic progenitor cells were generated using Protocol A (Chal et al. from Nature Protocols (2016)) and Protocol B (Xi et al. Cell Rep (2017)). (B) Culture-expanded iPSC-derived myogenic progenitor cells expressed MyHC (red) and MyoD (green).

 网站首页
网站首页