概要
MyeloCult™ M5300 is a culture medium for the initiation and maintenance of myeloid long-term cultures of mouse hematopoietic cells and stromal cell feeder layers. The sera used in this formulation have been pre-tested and selected for their ability to support long-term myelopoiesis by primitive mouse hematopoietic cells (e.g. in long-term culture-initiating cell [LTC-IC] assays).
MyeloCult™ M5300 requires the addition of freshly prepared Hydrocortisone (Catalog #07904).
MyeloCult™ M5300 requires the addition of freshly prepared Hydrocortisone (Catalog #07904).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | MyeloCult™ M5300 | 05350 | All | English |
| Product Information Sheet 2 | MyeloCult™ M5300 | 05350 | All | English |
| Manual | MyeloCult™ M5300 | 05300 | All | English |
| Safety Data Sheet | MyeloCult™ M5300 | 05350 | All | English |
数据及文献
Data
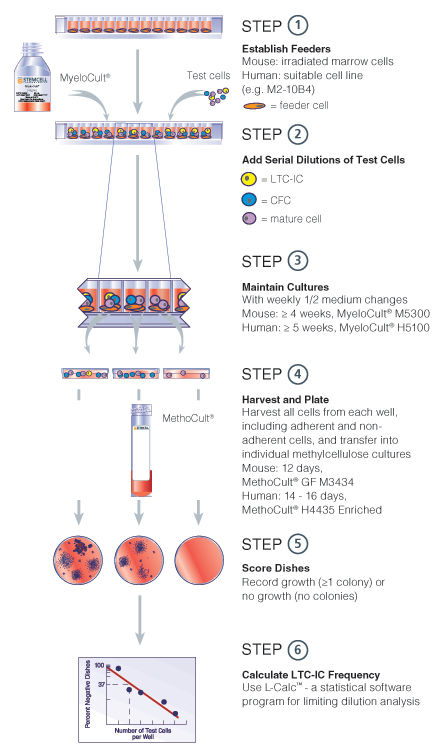
Figure 1. Limiting dilution LTC-IC assay
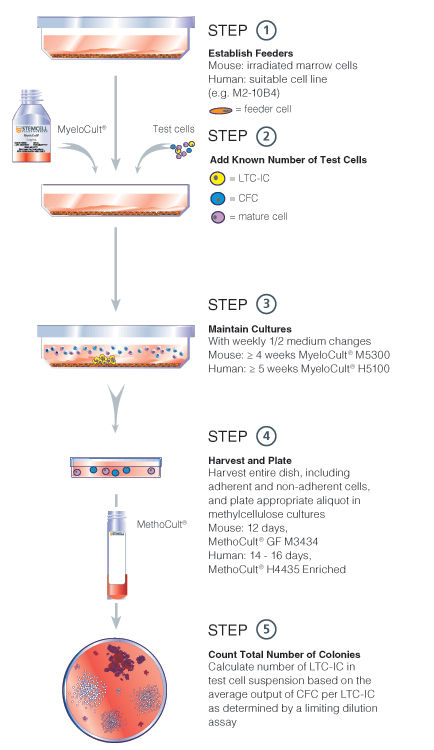
Figure 2. Bulk culture LTC-IC assay
Publications (50)
Cell stem cell 2020
Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency.
Abstract
Abstract
Quiescence is a fundamental property that maintains hematopoietic stem cell (HSC) potency throughout life. Quiescent HSCs are thought to rely on glycolysis for their energy, but the overall metabolic properties of HSCs remain elusive. Using combined approaches, including single-cell RNA sequencing (RNA-seq), we show that mitochondrial membrane potential (MMP) distinguishes quiescent from cycling-primed HSCs. We found that primed, but not quiescent, HSCs relied readily on glycolysis. Notably, in vivo inhibition of glycolysis enhanced the competitive repopulation ability of primed HSCs. We further show that HSC quiescence is maintained by an abundance of large lysosomes. Repression of lysosomal activation in HSCs led to further enlargement of lysosomes while suppressing glucose uptake. This also induced increased lysosomal sequestration of mitochondria and enhanced the competitive repopulation ability of primed HSCs by over 90-fold in vivo. These findings show that restraining lysosomal activity preserves HSC quiescence and potency and may be therapeutically relevant.
Nature communications 2018 JUN
miR-143/145 differentially regulate hematopoietic stem and progenitor activity through suppression of canonical TGFbeta$ signaling.
Abstract
Abstract
Expression of miR-143 and miR-145 is reduced in hematopoietic stem/progenitor cells (HSPCs) of myelodysplastic syndrome patients with a deletion in the long arm of chromosome 5. Here we show that mice lacking miR-143/145 have impaired HSPC activity with depletion of functional hematopoietic stem cells (HSCs), but activation of progenitor cells (HPCs). We identify components of the transforming growth factor beta$ (TGFbeta$) pathway as key targets of miR-143/145. Enforced expression of the TGFbeta$ adaptor protein and miR-145 target, Disabled-2 (DAB2), recapitulates the HSC defect seen in miR-143/145-/- mice. Despite reduced HSC activity, older miR-143/145-/- and DAB2-expressing mice show elevated leukocyte counts associated with increased HPC activity. A subset of mice develop a serially transplantable myeloid malignancy, associated with expansion of HPC. Thus, miR-143/145 play a cell context-dependent role in HSPC function through regulation of TGFbeta$/DAB2 activation, and loss of these miRNAs creates a preleukemic state.
The Journal of experimental medicine 2015 MAY
Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis.
Abstract
Abstract
Blood flow promotes emergence of definitive hematopoietic stem cells (HSCs) in the developing embryo, yet the signals generated by hemodynamic forces that influence hematopoietic potential remain poorly defined. Here we show that fluid shear stress endows long-term multilineage engraftment potential upon early hematopoietic tissues at embryonic day 9.5, an embryonic stage not previously described to harbor HSCs. Effects on hematopoiesis are mediated in part by a cascade downstream of wall shear stress that involves calcium efflux and stimulation of the prostaglandin E2 (PGE2)-cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling axis. Blockade of the PGE2-cAMP-PKA pathway in the aorta-gonad-mesonephros (AGM) abolished enhancement in hematopoietic activity. Furthermore, Ncx1 heartbeat mutants, as well as static cultures of AGM, exhibit lower levels of expression of prostaglandin synthases and reduced phosphorylation of the cAMP response element-binding protein (CREB). Similar to flow-exposed cultures, transient treatment of AGM with the synthetic analogue 16,16-dimethyl-PGE2 stimulates more robust engraftment of adult recipients and greater lymphoid reconstitution. These data provide one mechanism by which biomechanical forces induced by blood flow modulate hematopoietic potential.
Blood 2011 JUL
Crebbp haploinsufficiency in mice alters the bone marrow microenvironment, leading to loss of stem cells and excessive myelopoiesis.
Abstract
Abstract
CREB-binding protein (CREBBP) is important for the cell-autonomous regulation of hematopoiesis, including the stem cell compartment. In the present study, we show that CREBBP plays an equally pivotal role in microenvironment-mediated regulation of hematopoiesis. We found that the BM microenvironment of Crebbp(+/-) mice was unable to properly maintain the immature stem cell and progenitor cell pools. Instead, it stimulates myeloid differentiation, which progresses into a myeloproliferation phenotype. Alterations in the BM microenvironment resulting from haploinsufficiency of Crebbp included a marked decrease in trabecular bone that was predominantly caused by increased osteoclastogenesis. Although CFU-fibroblast (CFU-F) and total osteoblast numbers were decreased, the bone formation rate was similar to that found in wild-type mice. At the molecular level, we found that the known hematopoietic modulators matrix metallopeptidase-9 (MMP9) and kit ligand (KITL) were decreased with heterozygous levels of Crebbp. Lastly, potentially important regulatory proteins, endothelial cell adhesion molecule 1 (ESAM1) and cadherin 5 (CDH5), were increased on Crebbp(+/-) endothelial cells. Our findings reveal that a full dose of Crebbp is essential in the BM microenvironment to maintain proper hematopoiesis and to prevent excessive myeloproliferation.
Blood 2011 FEB
Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo.
Abstract
Abstract
Osteoblasts play a crucial role in the hematopoietic stem cell (HSC) niche; however, an overall increase in their number does not necessarily promote hematopoiesis. Because the activity of osteoblasts and osteoclasts is coordinately regulated, we hypothesized that active bone-resorbing osteoclasts would participate in HSC niche maintenance. Mice treated with bisphosphonates exhibited a decrease in proportion and absolute number of Lin(-)cKit(+)Sca1(+) Flk2(-) (LKS Flk2(-)) and long-term culture-initiating cells in bone marrow (BM). In competitive transplantation assays, the engraftment of treated BM cells was inferior to that of controls, confirming a decrease in HSC numbers. Accordingly, bisphosphonates abolished the HSC increment produced by parathyroid hormone. In contrast, the number of colony-forming-unit cells in BM was increased. Because a larger fraction of LKS in the BM of treated mice was found in the S/M phase of the cell cycle, osteoclast impairment makes a proportion of HSCs enter the cell cycle and differentiate. To prove that HSC impairment was a consequence of niche manipulation, a group of mice was treated with bisphosphonates and then subjected to BM transplantation from untreated donors. Treated recipient mice experienced a delayed hematopoietic recovery compared with untreated controls. Our findings demonstrate that osteoclast function is fundamental in the HSC niche.
The Journal of biological chemistry 2010 OCT
Vinculin is indispensable for repopulation by hematopoietic stem cells, independent of integrin function.
Abstract
Abstract
Vinculin is a highly conserved actin-binding protein that is localized in integrin-mediated focal adhesion complexes. Although critical roles have been proposed for integrins in hematopoietic stem cell (HSC) function, little is known about the involvement of intracellular focal adhesion proteins in HSC functions. This study showed that the ability of c-Kit(+)Sca1(+)Lin(-) HSCs to support reconstitution of hematopoiesis after competitive transplantation was severely impaired by lentiviral transduction with short hairpin RNA sequences for vinculin. The potential of these HSCs to differentiate into granulocytic and monocytic lineages, to migrate toward stromal cell-derived factor 1α, and to home to the bone marrow in vivo were not inhibited by the loss of vinculin. However, the capacities to form long term culture-initiating cells and cobblestone-like areas were abolished in vinculin-silenced c-Kit(+)Sca1(+)Lin(-) HSCs. In contrast, adhesion to the extracellular matrix was inhibited by silencing of talin-1, but not of vinculin. Whole body in vivo luminescence analyses to detect transduced HSCs confirmed the role of vinculin in long term HSC reconstitution. Our results suggest that vinculin is an indispensable factor determining HSC repopulation capacity, independent of integrin functions.

 网站首页
网站首页