概要
MethoCult™ M3334 is optimized for the growth and enumeration of erythroid progenitor cells (late BFU-E and CFU-E) in colony-forming unit (CFU) assays of mouse bone marrow, spleen, and fetal liver cells. This formulation contains erythropoietin (EPO) but does not contain other recombinant cytokines.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MethoCult™ M3334 | 03334 | All | English |
| Manual | MethoCult™ M3334 | 03334 | All | English |
| Safety Data Sheet | MethoCult™ M3334 | 03334 | All | English |
数据及文献
Data
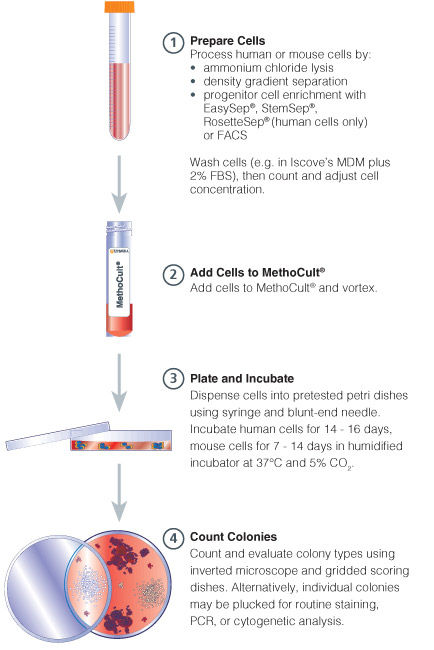
Figure 1. Procedure Summary for Hematopoietic CFU Assays
Publications (23)
Blood 2011 MAY
Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment.
Abstract
Abstract
Erythropoietin (Epo) has been used in the treatment of anemia resulting from numerous etiologies, including renal disease and cancer. However, its effects are controversial and the expression pattern of the Epo receptor (Epo-R) is debated. Using in vivo lineage tracing, we document that within the hematopoietic and mesenchymal lineage, expression of Epo-R is essentially restricted to erythroid lineage cells. As expected, adult mice treated with a clinically relevant dose of Epo had expanded erythropoiesis because of amplification of committed erythroid precursors. Surprisingly, we also found that Epo induced a rapid 26% loss of the trabecular bone volume and impaired B-lymphopoiesis within the bone marrow microenvironment. Despite the loss of trabecular bone, hematopoietic stem cell populations were unaffected. Inhibition of the osteoclast activity with bisphosphonate therapy blocked the Epo-induced bone loss. Intriguingly, bisphosphonate treatment also reduced the magnitude of the erythroid response to Epo. These data demonstrate a previously unrecognized in vivo regulatory network coordinating erythropoiesis, B-lymphopoiesis, and skeletal homeostasis. Importantly, these findings may be relevant to the clinical application of Epo.
Blood 2011 MAR
Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells.
Abstract
Abstract
Haploinsufficiency for ribosomal protein genes has been implicated in the pathophysiology of Diamond-Blackfan anemia (DBA) and the 5q-syndrome, a subtype of myelodysplastic syndrome. The p53 pathway is activated by ribosome dysfunction, but the molecular basis for selective impairment of the erythroid lineage in disorders of ribosome function has not been determined. We found that p53 accumulates selectively in the erythroid lineage in primary human hematopoietic progenitor cells after expression of shRNAs targeting RPS14, the ribosomal protein gene deleted in the 5q-syndrome, or RPS19, the most commonly mutated gene in DBA. Induction of p53 led to lineage-specific accumulation of p21 and consequent cell cycle arrest in erythroid progenitor cells. Pharmacologic inhibition of p53 rescued the erythroid defect, whereas nutlin-3, a compound that activates p53 through inhibition of HDM2, selectively impaired erythropoiesis. In bone marrow biopsies from patients with DBA or del(5q) myelodysplastic syndrome, we found an accumulation of nuclear p53 staining in erythroid progenitor cells that was not present in control samples. Our findings indicate that the erythroid lineage has a low threshold for the induction of p53, providing a basis for the failure of erythropoiesis in the 5q-syndrome, DBA, and perhaps other bone marrow failure syndromes.
Experimental biology and medicine (Maywood, N.J.) 2011 JUN
Protective effect of dammarane sapogenins against chemotherapy-induced myelosuppression in mice.
Abstract
Abstract
Chemotherapy is the most common way to treat malignancies, but myelosuppression, one of its common side-effects, is a formidable problem. The present study described the protective role of dammarane sapogenins (DS), an active fraction from oriental ginseng, on myelosuppression induced by cyclophosphamide (CP) in mice. DS was orally administered at different dosages (37.5, 75, and 150 mg/kg) for 10 d after CP administration (200 mg/kg intraperitoneally). The results showed that DS increased the number of white blood cells (WBC) on day 3 and day 7 (P textless 0.05), such that WBC levels were increased by 105.7 ± 29.5% at 75 mg/kg of DS on day 3 (P textless 0.05, compared with the CP group). Similar results were observed in red blood cells and platelets in DS-treated groups. The colony-forming assay demonstrated that the depressed numbers of CFU-GM (colony-forming unit-granulocyte and macrophage), CFU-E (colony-forming unit-erythroid), BFU-E (burst-forming unit-erythroid), CFU-Meg (colony-forming unit-megakaryocyte) and CFU-GEMM (colony-forming unit-granulocyte, -erythrocyte, -monocyte and -megakaryocyte) induced by CP were significantly reversed after DS treatment. Moreover, the ameliorative effect of DS on myelosuppression was also observed in the femur by hematoxylin/eosin staining. In DS-treated groups, ConA-induced splenocyte proliferation was enhanced significantly at all the doses (37.5, 75, 150 mg/kg) on day 3 at the rate of 50.3 ± 8.0%, 77.6 ± 8.5% and 44.5 ± 8.4%, respectively, while lipopolysaccharide-induced proliferation was increased mainly on day 7 (P textless 0.01), with an increased rate of 39.8 ± 5.6%, 34.9 ± 6.6% and 38.3 ± 7.3%, respectively. The thymus index was also markedly increased by 70.4% and 36.6% at 75 mg/kg on days 3 and 7, respectively, as compared with the CP group. In summary, DS has a protective function against CP-induced myelosuppression. Its mechanism might be related to stimulating hematopoiesis recovery, as well as enhancing the immunological function.
Blood 2011 JAN
Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche.
Abstract
Abstract
The physiologic roles of angiopoietin-like proteins (Angptls) in the hematopoietic system remain unknown. Here we show that hematopoietic stem cells (HSCs) in Angptl3-null mice are decreased in number and quiescence. HSCs transplanted into Angptl3-null recipient mice exhibited impaired repopulation. Bone marrow sinusoidal endothelial cells express high levels of Angptl3 and are adjacent to HSCs. Importantly, bone marrow stromal cells or endothelium deficient in Angptl3 have a significantly decreased ability to support the expansion of repopulating HSCs. Angptl3 represses the expression of the transcription factor Ikaros, whose unregulated overexpression diminishes the repopulation activity of HSCs. Angptl3, as an extrinsic factor, thus supports the stemness of HSCs in the bone marrow niche.
Blood 2011 JAN
Enhanced erythropoiesis in Hfe-KO mice indicates a role for Hfe in the modulation of erythroid iron homeostasis.
Abstract
Abstract
In hereditary hemochromatosis, mutations in HFE lead to iron overload through abnormally low levels of hepcidin. In addition, HFE potentially modulates cellular iron uptake by interacting with transferrin receptor, a crucial protein during erythropoiesis. However, the role of HFE in this process was never explored. We hypothesize that HFE modulates erythropoiesis by affecting dietary iron absorption and erythroid iron intake. To investigate this, we used Hfe-KO mice in conditions of altered dietary iron and erythropoiesis. We show that Hfe-KO mice can overcome phlebotomy-induced anemia more rapidly than wild-type mice (even when iron loaded). Second, we evaluated mice combining the hemochromatosis and β-thalassemia phenotypes. Our results suggest that lack of Hfe is advantageous in conditions of increased erythropoietic activity because of augmented iron mobilization driven by deficient hepcidin response. Lastly, we demonstrate that Hfe is expressed in erythroid cells and impairs iron uptake, whereas its absence exclusively from the hematopoietic compartment is sufficient to accelerate recovery from phlebotomy. In summary, we demonstrate that Hfe influences erythropoiesis by 2 distinct mechanisms: limiting hepcidin expression under conditions of simultaneous iron overload and stress erythropoiesis, and impairing transferrin-bound iron uptake by erythroid cells. Moreover, our results provide novel suggestions to improve the treatment of hemochromatosis.
Molecular and cellular biology 2010 OCT
The transcriptional mediator subunit MED1/TRAP220 in stromal cells is involved in hematopoietic stem/progenitor cell support through osteopontin expression.
Abstract
Abstract
MED1/TRAP220, a subunit of the transcriptional Mediator/TRAP complex, is crucial for various biological events through its interaction with distinct activators, such as nuclear receptors and GATA family activators. In hematopoiesis, MED1 plays a pivotal role in optimal nuclear receptor-mediated myelomonopoiesis and GATA-1-induced erythropoiesis. In this study, we present evidence that MED1 in stromal cells is involved in supporting hematopoietic stem and/or progenitor cells (HSPCs) through osteopontin (OPN) expression. We found that the proliferation of bone marrow (BM) cells cocultured with MED1 knockout (Med1(-/-)) mouse embryonic fibroblasts (MEFs) was significantly suppressed compared to the control. Furthermore, the number of long-term culture-initiating cells (LTC-ICs) was attenuated for BM cells cocultured with Med1(-/-) MEFs. The vitamin D receptor (VDR)- and Runx2-mediated expression of OPN, as well as Mediator recruitment to the Opn promoter, was specifically attenuated in the Med1(-/-) MEFs. Addition of OPN to these MEFs restored the growth of cocultured BM cells and the number of LTC-ICs, both of which were attenuated by the addition of the anti-OPN antibody to Med1(+/+) MEFs and to BM stromal cells. Consequently, MED1 in niche appears to play an important role in supporting HSPCs by upregulating VDR- and Runx2-mediated transcription on the Opn promoter.

 网站首页
网站首页