概要
MethoCult™ GF M3434 is optimized for the growth and enumeration of hematopoietic progenitor cells in colony-forming unit (CFU) assays of mouse bone marrow, spleen, peripheral blood, and fetal liver cells. MethoCult™ GF M3434 has been formulated to support optimal growth of primitive erythroid progenitor cells (BFU-E), granulocyte-macrophage progenitor cells (CFU-GM, CFU-G and CFU-M), and multi-potential granulocyte, erythroid, macrophage, megakaryocyte progenitor cells (CFU-GEMM). This formulation is compatible with STEMvision™ software for automated colony counting of mouse bone marrow CFU assays.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MethoCult™ GF M3434 | 03434, 03444 | All | English |
| Manual | MethoCult™ GF M3434 | 03434 | All | English |
| Safety Data Sheet | MethoCult™ GF M3434 | 03434, 03444 | All | English |
数据及文献
Data
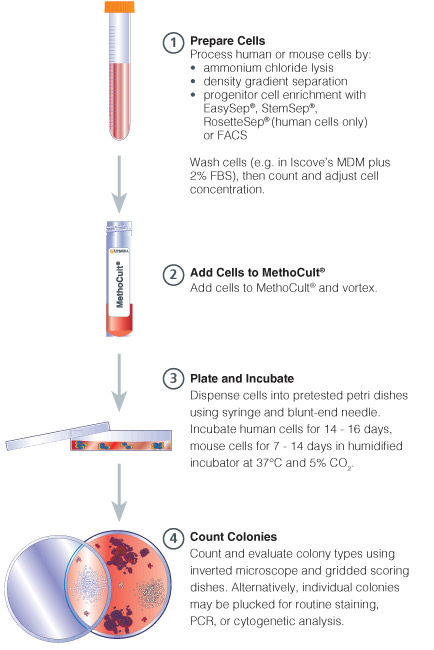
Figure 1. Procedure Summary for Hematopoietic CFU Assays
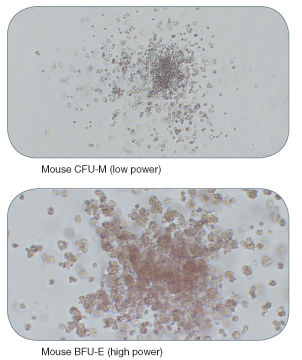
Figure 2. Examples of Colonies Derived from Mouse Hematopoietic Progenitors
Publications (167)
Cell stem cell 2020
Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency.
Abstract
Abstract
Quiescence is a fundamental property that maintains hematopoietic stem cell (HSC) potency throughout life. Quiescent HSCs are thought to rely on glycolysis for their energy, but the overall metabolic properties of HSCs remain elusive. Using combined approaches, including single-cell RNA sequencing (RNA-seq), we show that mitochondrial membrane potential (MMP) distinguishes quiescent from cycling-primed HSCs. We found that primed, but not quiescent, HSCs relied readily on glycolysis. Notably, in vivo inhibition of glycolysis enhanced the competitive repopulation ability of primed HSCs. We further show that HSC quiescence is maintained by an abundance of large lysosomes. Repression of lysosomal activation in HSCs led to further enlargement of lysosomes while suppressing glucose uptake. This also induced increased lysosomal sequestration of mitochondria and enhanced the competitive repopulation ability of primed HSCs by over 90-fold in vivo. These findings show that restraining lysosomal activity preserves HSC quiescence and potency and may be therapeutically relevant.
Cell stem cell 2019 mar
The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance.
Abstract
Abstract
It has been recently shown that increased oxidative phosphorylation, as reflected by increased mitochondrial activity, together with impairment of the mitochondrial stress response, can severely compromise hematopoietic stem cell (HSC) regeneration. Here we show that the NAD+-boosting agent nicotinamide riboside (NR) reduces mitochondrial activity within HSCs through increased mitochondrial clearance, leading to increased asymmetric HSC divisions. NR dietary supplementation results in a significantly enlarged pool of progenitors, without concurrent HSC exhaustion, improves survival by 80{\%}, and accelerates blood recovery after murine lethal irradiation and limiting-HSC transplantation. In immune-deficient mice, NR increased the production of human leucocytes from hCD34+ progenitors. Our work demonstrates for the first time a positive effect of NAD+-boosting strategies on the most primitive blood stem cells, establishing a link between HSC mitochondrial stress, mitophagy, and stem-cell fate decision, and unveiling the potential of NR to improve recovery of patients suffering from hematological failure including post chemo- and radiotherapy.
Cell stem cell 2019 jul
Targeting the RNA m6A Reader YTHDF2 Selectively Compromises Cancer Stem Cells in Acute Myeloid Leukemia.
Abstract
Abstract
Acute myeloid leukemia (AML) is an aggressive clonal disorder of hematopoietic stem cells (HSCs) and primitive progenitors that blocks their myeloid differentiation, generating self-renewing leukemic stem cells (LSCs). Here, we show that the mRNA m6A reader YTHDF2 is overexpressed in a broad spectrum of human AML and is required for disease initiation as well as propagation in mouse and human AML. YTHDF2 decreases the half-life of diverse m6A transcripts that contribute to the overall integrity of LSC function, including the tumor necrosis factor receptor Tnfrsf2, whose upregulation in Ythdf2-deficient LSCs primes cells for apoptosis. Intriguingly, YTHDF2 is not essential for normal HSC function, with YTHDF2 deficiency actually enhancing HSC activity. Thus, we identify YTHDF2 as a unique therapeutic target whose inhibition selectively targets LSCs while promoting HSC expansion.
Leukemia 2019 jul
Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis.
Abstract
Abstract
Clonal hematopoiesis (CH) is a common aging-associated condition with increased risk of hematologic malignancy. Knowledge of the mechanisms driving evolution from CH to overt malignancy has been hampered by a lack of in vivo models that orthogonally activate mutant alleles. Here, we develop independently regulatable mutations in DNA methyltransferase 3A (Dnmt3a) and nucleophosmin 1 (Npm1), observed in human CH and AML, respectively. We find Dnmt3a mutation expands hematopoietic stem and multipotent progenitor cells (HSC/MPPs), modeling CH. Induction of mutant Npm1 after development of Dnmt3a-mutant CH causes progression to myeloproliferative disorder (MPD), and more aggressive MPD is observed with longer latency between mutations. MPDs uniformly progress to acute myeloid leukemia (AML) following transplant, accompanied by a decrease in HSC/MPPs and an increase in myeloid-restricted progenitors, the latter of which propagate AML in tertiary recipient mice. At a molecular level, progression of CH to MPD is accompanied by selection for mutations activating Ras/Raf/MAPK signaling. Progression to AML is characterized by additional oncogenic signaling mutations (Ptpn11, Pik3r1, Flt3) and/or mutations in epigenetic regulators (Hdac1, Idh1, Arid1a). Together, our study demonstrates that Npm1 mutation drives evolution of Dnmt3a-mutant CH to AML and rate of disease progression is accelerated with longer latency of CH.
Nature communications 2019 aug
PTPsigma inhibitors promote hematopoietic stem cell regeneration.
Abstract
Abstract
Receptor type protein tyrosine phosphatase-sigma (PTPsigma) is primarily expressed by adult neurons and regulates neural regeneration. We recently discovered that PTPsigma is also expressed by hematopoietic stem cells (HSCs). Here, we describe small molecule inhibitors of PTPsigma that promote HSC regeneration in vivo. Systemic administration of the PTPsigma inhibitor, DJ001, or its analog, to irradiated mice promotes HSC regeneration, accelerates hematologic recovery, and improves survival. Similarly, DJ001 administration accelerates hematologic recovery in mice treated with 5-fluorouracil chemotherapy. DJ001 displays high specificity for PTPsigma and antagonizes PTPsigma via unique non-competitive, allosteric binding. Mechanistically, DJ001 suppresses radiation-induced HSC apoptosis via activation of the RhoGTPase, RAC1, and induction of BCL-XL. Furthermore, treatment of irradiated human HSCs with DJ001 promotes the regeneration of human HSCs capable of multilineage in vivo repopulation. These studies demonstrate the therapeutic potential of selective, small-molecule PTPsigma inhibitors for human hematopoietic regeneration.
Cell stem cell 2019 aug
Interconversion between Tumorigenic and Differentiated States in Acute Myeloid Leukemia.
Abstract
Abstract
Tumors are composed of phenotypically heterogeneous cancer cells that often resemble various differentiation states of their lineage of origin. Within this hierarchy, it is thought that an immature subpopulation of tumor-propagating cancer stem cells (CSCs) differentiates into non-tumorigenic progeny, providing a rationale for therapeutic strategies that specifically eradicate CSCs or induce their differentiation. The clinical success of these approaches depends on CSC differentiation being unidirectional rather than reversible, yet this question remains unresolved even in prototypically hierarchical malignancies, such as acute myeloid leukemia (AML). Here, we show in murine and human models of AML that, upon perturbation of endogenous expression of the lineage-determining transcription factor PU.1 or withdrawal of established differentiation therapies, some mature leukemia cells can de-differentiate and reacquire clonogenic and leukemogenic properties. Our results reveal plasticity of CSC maturation in AML, highlighting the need to therapeutically eradicate cancer cells across a range of differentiation states.

 网站首页
网站首页