概要
MesenCult™ Expansion Kit (Mouse) is standardized for the culture of mouse mesenchymal stromal cells (MSCs; also known as mesenchymal stem cells) and mouse embryonic fibroblasts (MEFs). The kit includes MesenCult™ Basal Medium (Mouse), MesenCult™ 10X Supplement (Mouse), and MesenPure™. MesenCult™ Expansion Medium has been optimized for the derivation and expansion of mouse MSCs and MEFs in vitro as well as for the detection of colony-forming unit–fibroblasts (CFUF). This kit was optimized using cells from the mouse strain C57BL/6.
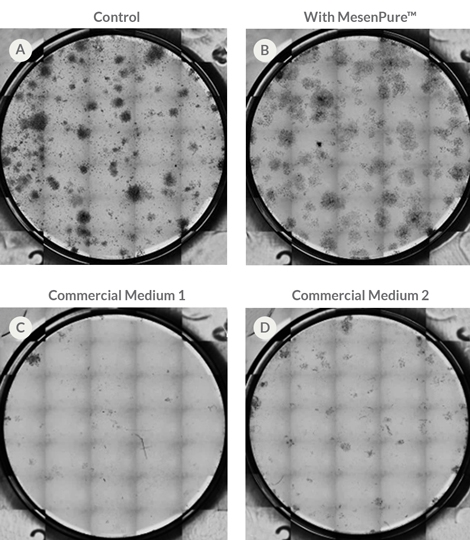
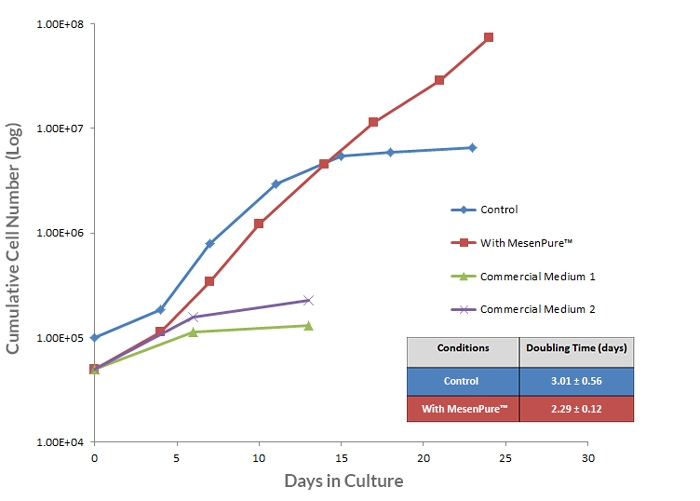
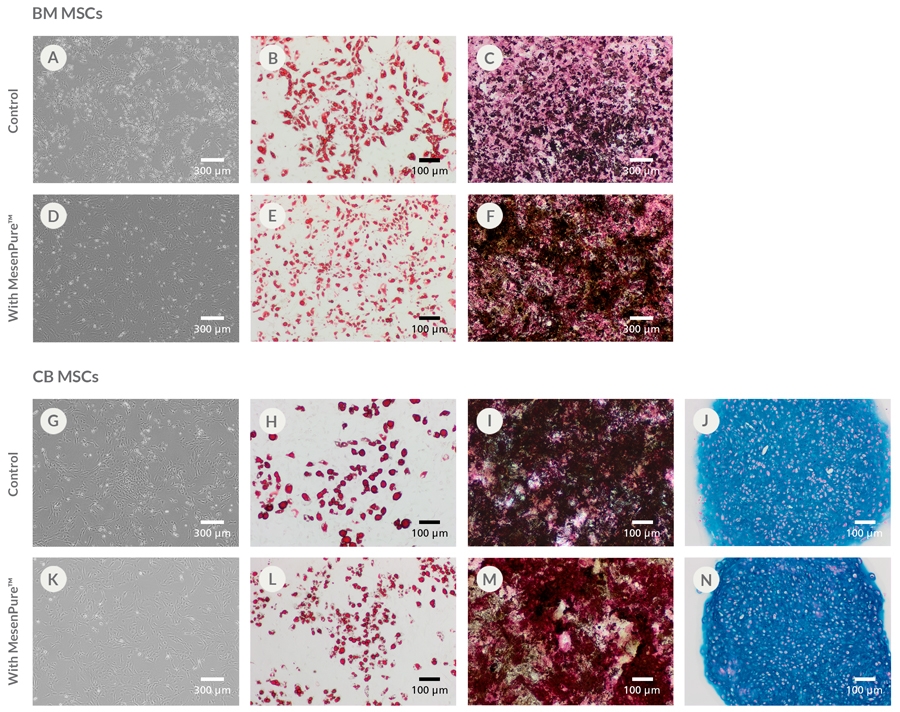
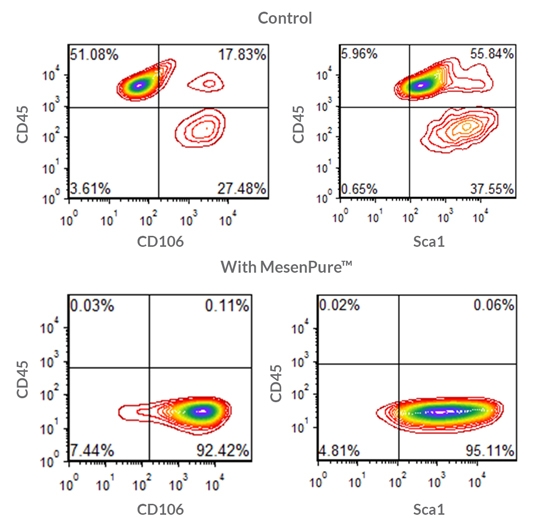
To facilitate the enrichment of MSCs and MEFs during cell culture without serial passaging and frequent medium changes, simply add MesenPure™ to complete MesenCult™ Expansion Medium just prior to use. Although not required, the addition of MesenPure™ is strongly recommended, as the resulting MSC and MEF cultures are more homogeneous and exhibit more robust proliferation, differentiation, and colony formation when compared to complete MesenCult™ Expansion Medium alone.
NOTE: MesenCult™ Expansion Medium must be supplemented with L-Glutamine (Catalog #07100).
数据及文献
Publications (16)
Cell stem cell 2021 feb
Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging.
P. Deng et al.
Abstract
Skeletal aging is a complex process, characterized by a decrease in bone formation, an increase in marrow fat, and stem cell exhaustion. Loss of H3K9me3, a heterochromatin mark, has been proposed to be associated with aging. Here, we report that loss of KDM4B in mesenchymal stromal cells (MSCs) exacerbated skeletal aging and osteoporosis by reducing bone formation and increasing marrow adiposity via increasing H3K9me3. KDM4B epigenetically coordinated $\beta$-catenin/Smad1-mediated transcription by removing repressive H3K9me3. Importantly, KDM4B ablation impaired MSC self-renewal and promoted MSC exhaustion by inducing senescence-associated heterochromatin foci formation, providing a mechanistic explanation for stem cell exhaustion with aging. Moreover, while KDM4B was required for parathyroid hormone-mediated bone anabolism, KDM4B depletion accelerated bone loss and marrow adiposity induced by a high-fat diet. Our results suggest that the epigenetic rejuvenation and reversing bone-fat imbalance might be new strategies for preventing and treating skeletal aging and osteoporosis by activating KDM4B in MSCs.
Stem cell reviews and reports 2020 may
Aging-Related Reduced Expression of CXCR4 on Bone Marrow Mesenchymal Stromal Cells Contributes to Hematopoietic Stem and Progenitor Cell Defects.
P. Singh et al.
Abstract
Aging impairs the regenerative potential of hematopoietic stem cells (HSC) and skews differentiation towards the myeloid lineage. The bone marrow (BM) microenvironment has recently been suggested to influence HSC aging, however the mechanisms whereby BM stromal cells mediate this effect is unknown. Here we show that aging-associated decreased expression of CXCR4 expression on BM mesenchymal stem cells (MSC) plays a crucial role in the development of the hematopoietic stem and progenitor cells (HSPC) aging phenotype. The BM MSC from old mice was sufficient to drive a premature aging phenotype of young HSPC when cultured together ex vivo. The impaired ability of old MSC to support HSPC function is associated with reduced expression of CXCR4 on BM MSC of old mice. Deletion of the CXCR4 gene in young MSC accelerates an aging phenotype in these cells characterized by increased production of reactive oxygen species (ROS), DNA damage, senescence, and reduced proliferation. Culture of HSPC from young mice with CXCR4 deficient MSC also from young mice led to a premature aging phenotype in the young HSPC, as evidenced by reduced hematopoietic regeneration and enhanced myeloid differentiation. Mechanistically, CXCR4 signaling prevents BM MSC dysfunction by suppressing oxidative stress, as treatment of old or CXCR4 deficient MSC with N-acetyl-L-cysteine (NAC), improved their niche supporting activity, and attenuated the HSPC aging phenotype. Our studies suggest that age-associated reduction in CXCR4 expression on BM MSC impairs hematopoietic niche activity with increased ROS production, driving an HSC aging phenotype. Thus, modulation of the SDF-1/CXCR4 axis in MSC may lead to novel interventions to alleviate the age-associated decline in immune/hematopoietic function.
Journal of biomedical materials research. Part A 2020 jun
Increased yield of gelatin coated therapeutic cells through cholesterol insertion.
K. A. Davis et al.
Abstract
Gelatin coatings are effective in increasing the retention of MSCs injected into the heart and minimizing the damage from acute myocardial infarction (AMI), but early studies suffered from low fractions of the MSCs coated with gelatin. Biotinylation of the MSC surface is a critical first step in the gelatin coating process, and in this study, we evaluated the use of biotinylated cholesterol lipid insertion" anchors as a substitute for the covalent NHS-biotin anchors to the cell surface. Streptavidin-eosin molecules where eosin is our photoinitiator can then be bound to the cell surface through biotin-streptavidin affinity. The use of cholesterol anchors increased streptavidin density on the surface of MSCs further driving polymerization and allowing for an increased fraction of MSCs coated with gelatin (83{\%}) when compared to NHS-biotin (52{\%}). Additionally the cholesterol anchors increased the uniformity of the coating on the MSC surface and supported greater numbers of coated MSCs even when the streptavidin density was slightly lower than that of an NHS-biotin anchoring strategy. Critically this improvement in gelatin coating efficiency did not impact cytokine secretion and other critical MSC functions. Proper selection of the cholesterol anchor and the biotinylation conditions supports cellular function and densities of streptavidin on the MSC surface of up to {\~{}}105 streptavidin molecules/$\mu$m2 . In all these cholesterol anchors offer an effective path towards the formation of conformal coatings on the majority of MSCs to improve the retention of MSCs in the heart following AMI."
Pharmacological research 2020 jul
Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer.
T. Li et al.
Abstract
Both antitumor and protumor property of mesenchymal stem cells (MSCs) have been demonstrated. We hypothesize that this contradiction is due to the heterogeneity of MSC subsets and that extracellular vesicles (EVs) from distinct MSC subsets can transfer the corresponding antitumor activities. Here we evaluated the antitumor activities of two subsets of adipose-derived mesenchymal stem cells (ADSCs) and ADSC-derived EVs (ADSC-EVs) in immunocompetent syngeneic mouse models of breast cancer. We identified CD90high and CD90low ADSC subsets and demonstrated that CD90high ADSCs could be converted into CD90low ADSCs by stimulation with LPS. CD90low ADSCs and its derived EVs significantly inhibited tumor growth in tumor-bearing mice. Benefit of tumor control were associated with decreased tumor cell proliferation and migration, and enhanced tumor cell apoptosis mediated by ADSC-EVs. Antioncogenic miRNA-16-5p loaded CD90low ADSC-EVs further significantly enhanced antitumor activities. Taken together, this study represents the first attempt to apply our newly identified antitumor ADSCs and its derived EVs in preclinical treatment of breast cancer. This study also provides the evidence that EVs can serve as a novel and effective therapeutics or drug delivery vesicle. This new therapeutic approach could be potentially applicable to breast cancer and many other types of cancer.
Nature communications 2020 jan
Gastric squamous-columnar junction contains a large pool of cancer-prone immature osteopontin responsive Lgr5-CD44+ cells.
D.-J. Fu et al.
Abstract
Areas of a junction between two types of epithelia are known to be cancer-prone in many organ systems. However, mechanisms for preferential malignant transformation at the junction areas remain insufficiently elucidated. Here we report that inactivation of tumor suppressor genes Trp53 and Rb1 in the gastric squamous-columnar junction (SCJ) epithelium results in preferential formation of metastatic poorly differentiated neoplasms, which are similar to human gastroesophageal carcinoma. Unlike transformation-resistant antral cells, SCJ cells contain a highly proliferative pool of immature Lgr5-CD44+ cells, which are prone to transformation in organoid assays, comprise early dysplastic lesions, and constitute up to 30{\%} of all neoplastic cells. CD44 ligand osteopontin (OPN) is preferentially expressed in and promotes organoid formation ability and transformation of the SCJ glandular epithelium. OPN and CD44 overexpression correlate with the worst prognosis of human gastroesophageal carcinoma. Thus, detection and selective targeting of the active OPN-CD44 pathway may have direct clinical relevance.
Bioactive materials 2020 dec
3D-printable supramolecular hydrogels with shear-thinning property: fabricating strength tunable bioink via dual crosslinking.
T. Hu et al.
Abstract
3-dimensional (3D) bioprinting technology provides promising strategy in the fabrication of artificial tissues and organs. As the fundamental element in bioprinting process, preparation of bioink with ideal mechanical properties without sacrifice of biocompatibility is a great challenge. In this study, a supramolecular hydrogel-based bioink is prepared by polyethylene glycol (PEG) grafted chitosan, $\alpha$-cyclodextrin ($\alpha$-CD) and gelatin. It has a primary crosslinking structure through the aggregation of the pseudo-polyrotaxane-like side chains, which are formed from the host-guest interactions between $\alpha$-CD and PEG side chain. Apparent viscosity measurement shows the shear-shinning property of this bioink, which might be due to the reversibility of the physical crosslinking. Moreover, with $\beta$-glycerophosphate at different concentrations as the secondary crosslinking agent, the printed constructs demonstrate different Young's modulus (p {\textless} 0.001). They could also maintain the Young's modulus in cell culture condition for at least 21 days (p {\textless} 0.05). By co-culturing each component with fibroblasts, CCK-8 assay demonstrate cellular viability is higher than 80{\%}. After bioprinting and culturing, immunofluorescence staining with quantification indicate the expression of Ki-67, Paxillin, and N-cadherin is higher in day 14 than those in day 3 (p {\textless} 0.05). Oil red O and Nissl body specific staining reflect strength tunable bioink may have impact on the cell fate of mesenchymal stem cells (p {\textless} 0.05). This work might provide new idea for advanced bioink in the application of re-establishing complicated tissues and organs.
View All Publications

 网站首页
网站首页