概要
MegaCult™-C Collagen and Medium Without Cytokines kit contains medium and collagen solution for colony-forming unit (CFU) assays of human or mouse megakaryocyte progenitor cells (CFU-Mk). The addition of cytokines is required.
MegaCult™-C medium is intended for the culture of CFU-Mk in human bone marrow, mobilized peripheral blood, and cord blood samples, after addition of appropriate cytokines. It is suitable for use with CD34+ enriched cells, mononuclear cells, and cells isolated by other purification methods. It is also intended for assays of megakaryocyte progenitor cells in unseparated or purified cell suspensions of mouse bone marrow, after addition of appropriate cytokines.
MegaCult™-C medium is intended for the culture of CFU-Mk in human bone marrow, mobilized peripheral blood, and cord blood samples, after addition of appropriate cytokines. It is suitable for use with CD34+ enriched cells, mononuclear cells, and cells isolated by other purification methods. It is also intended for assays of megakaryocyte progenitor cells in unseparated or purified cell suspensions of mouse bone marrow, after addition of appropriate cytokines.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | MegaCult™-C Collagen and Medium Without Cytokines | 04960 | All | English |
| Product Information Sheet 2 | MegaCult™-C Collagen and Medium Without Cytokines | 04960 | All | English |
| Product Information Sheet | MegaCult™-C Medium Without Cytokines | 04900 | All | English |
| Product Information Sheet | Collagen Solution | 04902 | All | English |
| Manual | MegaCult™-C Collagen and Medium Without Cytokines | 04960 | All | English |
| Safety Data Sheet 1 | MegaCult™-C Collagen and Medium Without Cytokines | 04960 | All | English |
| Safety Data Sheet 2 | MegaCult™-C Collagen and Medium Without Cytokines | 04960 | All | English |
| Safety Data Sheet | Collagen Solution | 04902 | All | English |
数据及文献
Data
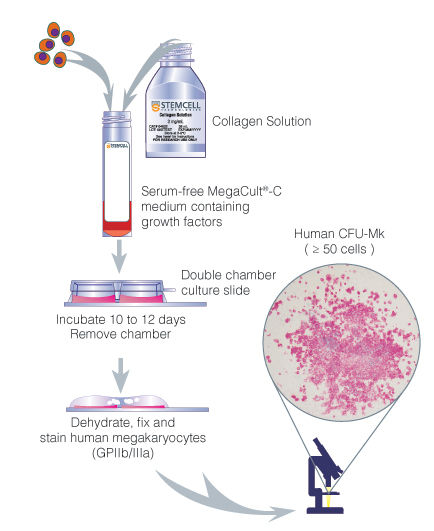
Figure 1. Procedure Summary for Assays of Human Megakaryocytic Progenitors
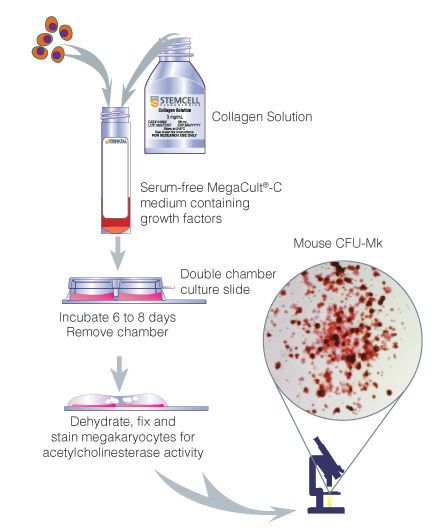
Figure 2. Procedure Summary for Assays of Mouse Megakaryocytic Progenitors
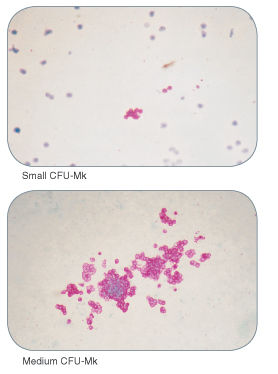
Figure 3. Examples of Colonies Derived From Human Megakaryocyte Progenitors
Publications (30)
Blood 2011 MAY
The EMT regulator Zeb2/Sip1 is essential for murine embryonic hematopoietic stem/progenitor cell differentiation and mobilization.
Abstract
Abstract
Zeb2 (Sip1/Zfhx1b) is a member of the zinc-finger E-box-binding (ZEB) family of transcriptional repressors previously demonstrated to regulate epithelial-to-mesenchymal transition (EMT) processes during embryogenesis and tumor progression. We found high Zeb2 mRNA expression levels in HSCs and hematopoietic progenitor cells (HPCs), and examined Zeb2 function in hematopoiesis through a conditional deletion approach using the Tie2-Cre and Vav-iCre recombination mouse lines. Detailed cellular analysis demonstrated that Zeb2 is dispensable for hematopoietic cluster and HSC formation in the aorta-gonadomesonephros region of the embryo, but is essential for normal HSC/HPC differentiation. In addition, Zeb2-deficient HSCs/HPCs fail to properly colonize the fetal liver and/or bone marrow and show enhanced adhesive properties associated with increased β1 integrin and Cxcr4 expression. Moreover, deletion of Zeb2 resulted in embryonic (Tie2-Cre) and perinatal (Vav-icre) lethality due to severe cephalic hemorrhaging and decreased levels of angiopoietin-1 and, subsequently, improper pericyte coverage of the cephalic vasculature. These results reveal essential roles for Zeb2 in embryonic hematopoiesis and are suggestive of a role for Zeb2 in hematopoietic-related pathologies in the adult.
Blood 2010 DEC
Expression level and differential JAK2-V617F-binding of the adaptor protein Lnk regulates JAK2-mediated signals in myeloproliferative neoplasms.
Abstract
Abstract
Activating mutations in signaling molecules, such as JAK2-V617F, have been associated with myeloproliferative neoplasms (MPNs). Mice lacking the inhibitory adaptor protein Lnk display deregulation of thrombopoietin/thrombopoietin receptor signaling pathways and exhibit similar myeloproliferative characteristics to those found in MPN patients, suggesting a role for Lnk in the molecular pathogenesis of these diseases. Here, we showed that LNK levels are up-regulated and correlate with an increase in the JAK2-V617F mutant allele burden in MPN patients. Using megakaryocytic cells, we demonstrated that Lnk expression is regulated by the TPO-signaling pathway, thus indicating an important negative control loop in these cells. Analysis of platelets derived from MPN patients and megakaryocytic cell lines showed that Lnk can interact with JAK2-WT and V617F through its SH2 domain, but also through an unrevealed JAK2-binding site within its N-terminal region. In addition, the presence of the V617F mutation causes a tighter association with Lnk. Finally, we found that the expression level of the Lnk protein can modulate JAK2-V617F-dependent cell proliferation and that its different domains contribute to the inhibition of multilineage and megakaryocytic progenitor cell growth in vitro. Together, our results indicate that changes in Lnk expression and JAK2-V617F-binding regulate JAK2-mediated signals in MPNs.
Blood 2009 MAR
Role for MKL1 in megakaryocytic maturation.
Abstract
Abstract
Megakaryoblastic leukemia 1 (MKL1), identified as part of the t(1;22) translocation specific to acute megakaryoblastic leukemia, is highly expressed in differentiated muscle cells and promotes muscle differentiation by activating serum response factor (SRF). Here we show that Mkl1 expression is up-regulated during murine megakaryocytic differentiation and that enforced overexpression of MKL1 enhances megakaryocytic differentiation. When the human erythroleukemia (HEL) cell line is induced to differentiate with 12-O-tetradecanoylphorbol 13-acetate, overexpression of MKL1 results in an increased number of megakaryocytes with a concurrent increase in ploidy. MKL1 overexpression also promotes megakaryocytic differentiation of primary human CD34(+) cells cultured in the presence of thrombopoietin. The effect of MKL1 is abrogated when SRF is knocked down, suggesting that MKL1 acts through SRF. Consistent with these findings in human cells, knockout of Mkl1 in mice leads to reduced platelet counts in peripheral blood, and reduced ploidy in bone marrow megakaryocytes. In conclusion, MKL1 promotes physiologic maturation of human and murine megakaryocytes.
Toxicology in vitro : an international journal published in association with BIBRA 2009 FEB
Application of human CFU-Mk assay to predict potential thrombocytotoxicity of drugs.
Abstract
Abstract
Megakaryocytopoiesis gives rise to platelets by proliferation and differentiation of lineage-specific progenitors, identified in vitro as Colony Forming Unit-Megakaryocytes (CFU-Mk). The aim of this study was to refine and optimize the in vitro Standard Operating Procedure (SOP) of the CFU-Mk assay for detecting drug-induced thrombocytopenia and to prevalidate a model for predicting the acute exposure levels that cause maximum tolerated decreases in the platelets count, based on the correlation with the maximal plasma concentrations (C max) in vivo. The assay was linear under the SOP conditions, and the in vitro endpoints (percentage of colonies growing) were reproducible within and across laboratories. The protocol performance phase was carried out testing 10 drugs (selected on the base of their recognised or potential in vivo haematotoxicity, according to the literature). Results showed that a relationship can be established between the maximal concentration in plasma (C max) and the in vitro concentrations that inhibited the 10-50-90 percent of colonies growth (ICs). When C max is lower than IC10, it is possible to predict that the chemicals have no direct toxicity effect on CFU-Mk and could not induce thrombocytopenia due to bone marrow damage. When the C max is higher than IC90 and/or IC50, thrombocytopenia can occur due to direct toxicity of chemicals on CFU-Mk progenitors.
Blood 2009 FEB
Incomplete restoration of Mpl expression in the mpl-/- mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis.
Abstract
Abstract
Expression of Mpl is restricted to hematopoietic cells in the megakaryocyte lineage and to undifferentiated progenitors, where it initiates critical cell survival and proliferation signals after stimulation by its ligand, thrombopoietin (TPO). As a result, a deficiency in Mpl function in patients with congenital amegakaryocytic thrombocytopenia (CAMT) and in mpl(-/-) mice produces profound thrombocytopenia and a severe stem cell-repopulating defect. Gene therapy has the potential to correct the hematopoietic defects of CAMT by ectopic gene expression that restores normal Mpl receptor activity. We rescued the mpl(-/-) mouse with a transgenic vector expressing mpl from the promoter elements of the 2-kb region of DNA just proximal to the natural gene start site. Transgene rescued mice exhibit thrombocytosis but only partial correction of the stem cell defect. Furthermore, they show very low-level expression of Mpl on platelets and megakaryocytes, and the transgene-rescued megakaryocytes exhibit diminished TPO-dependent kinase phosphorylation and reduced platelet production in bone marrow chimeras. Thrombocytosis is an unexpected consequence of reduced Mpl expression and activity. However, impaired TPO homeostasis in the transgene-rescued mice produces elevated plasma TPO levels, which serves as an unchecked stimulus to drive the observed excessive megakaryocytopoiesis.
Blood 2009 APR
Mef2C is a lineage-restricted target of Scl/Tal1 and regulates megakaryopoiesis and B-cell homeostasis.
Abstract
Abstract
The basic helix-loop-helix transcription factor stem cell leukemia gene (Scl) is a master regulator for hematopoiesis essential for hematopoietic specification and proper differentiation of the erythroid and megakaryocyte lineages. However, the critical downstream targets of Scl remain undefined. Here, we identified a novel Scl target gene, transcription factor myocyte enhancer factor 2 C (Mef2C) from Scl(fl/fl) fetal liver progenitor cell lines. Analysis of Mef2C(-/-) embryos showed that Mef2C, in contrast to Scl, is not essential for specification into primitive or definitive hematopoietic lineages. However, adult VavCre(+)Mef2C(fl/fl) mice exhibited platelet defects similar to those observed in Scl-deficient mice. The platelet counts were reduced, whereas platelet size was increased and the platelet shape and granularity were altered. Furthermore, megakaryopoiesis was severely impaired in vitro. Chromatin immunoprecipitation microarray hybridization analysis revealed that Mef2C is directly regulated by Scl in megakaryocytic cells, but not in erythroid cells. In addition, an Scl-independent requirement for Mef2C in B-lymphoid homeostasis was observed in Mef2C-deficient mice, characterized as severe age-dependent reduction of specific B-cell progenitor populations reminiscent of premature aging. In summary, this work identifies Mef2C as an integral member of hematopoietic transcription factors with distinct upstream regulatory mechanisms and functional requirements in megakaryocyte and B-lymphoid lineages.

 网站首页
网站首页