概要
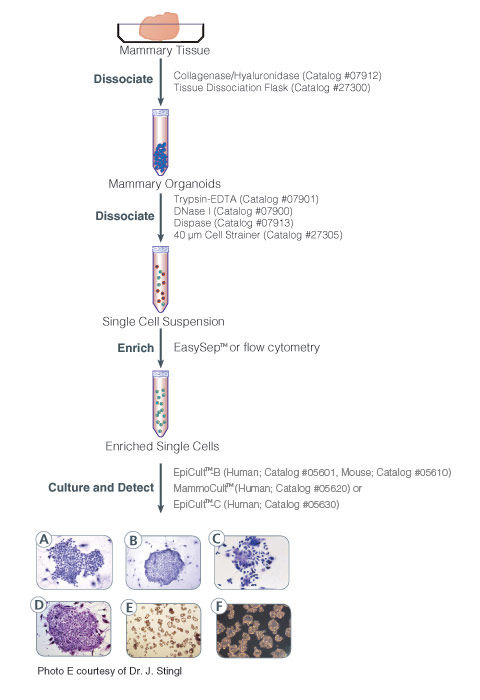
MammoCult™ Medium (Human) is a serum-free culture medium optimized for the culture of mammospheres from normal human primary breast tissues and tumorspheres from human breast cancer cell lines. For preparation of complete MammoCult™ Medium, Hydrocortisone Stock Solution (Catalog #07925) and Heparin Solution (Catalog #07980) are also required.
数据及文献
Publications (85)
The Biochemical journal 2016 MAY
Selenophosphate Synthetase 1 is an Essential Protein with Roles in Regulation of Redox Homeostasis in Mammals.
Tobe R et al.
Abstract
Selenophosphate synthetase (SPS) was initially detected in bacteria and was shown to synthesize selenophosphate, the active selenium donor. However, mammals have two SPS paralogs, which are designated SPS1 and SPS2. Although it is known that SPS2 catalyzes the synthesis of selenophosphate, the function of SPS1 remains largely unclear. To examine the role of SPS1 in mammals, we generated a Sps1 knockout mouse and found that systemic SPS1 deficiency led to embryos that were clearly underdeveloped by E8.5 and virtually resorbed by E14.5. The knockout of Sps1 in the liver preserved viability, but significantly affected the expression of a large number of mRNAs involved in cancer, embryonic development, and the glutathione system. Particularly notable was the extreme deficiency of glutaredoxin 1 (GLRX1) and glutathione-S-transferase omega 1. To assess these phenotypes at the cellular level, we targeted the removal of SPS1 in F9 cells, a mouse embryonal carcinoma cell line, which affected the glutathione system proteins and accordingly led to the accumulation of hydrogen peroxide in the cell. Further, we found that several malignant characteristics of SPS1-deficient F9 cells were reversed, suggesting that SPS1 played a role in supporting and/or sustaining cancer. In addition, the overexpression of mouse or human GLRX1 led to a reversal of observed increases in reactive oxygen species (ROS) in the F9 SPS1/GLRX1-deficient cells and resulted in levels that were similar to those in F9 SPS1-sufficient cells. The results suggested that SPS1 is an essential mammalian enzyme with roles in regulating redox homeostasis and controlling cell growth.
British journal of cancer 2016 MAY
Regulation of the T-box transcription factor Tbx3 by the tumour suppressor microRNA-206 in breast cancer.
Amir S et al.
Abstract
BACKGROUND The Tbx3 transcription factor is over-expressed in breast cancer, where it has been implicated in proliferation, migration and regulation of the cancer stem cell population. The mechanisms that regulate Tbx3 expression in cancer have not been fully explored. In this study, we demonstrate that Tbx3 is repressed by the tumour suppressor miR-206 in breast cancer cells. METHODS Bioinformatics prediction programmes and luciferase reporter assays were used to demonstrate that miR-206 negatively regulates Tbx3. We examined the impact of miR-206 on Tbx3 expression in breast cancer cells using miR-206 mimic and inhibitor. Gene/protein expression was examined by quantitative reverse-transcription-PCR and immunoblotting. The effects of miR-206 and Tbx3 on apoptosis, proliferation, invasion and cancer stem cell population was investigated by cell-death detection, colony formation, 3D-Matrigel and tumorsphere assays. RESULTS In this study, we examined the regulation of Tbx3 by miR-206. We demonstrate that Tbx3 is directly repressed by miR-206, and that this repression of Tbx3 is necessary for miR-206 to inhibit breast tumour cell proliferation and invasion, and decrease the cancer stem cell population. Moreover, Tbx3 and miR-206 expression are inversely correlated in human breast cancer. Kaplan-Meier analysis indicates that patients exhibiting a combination of high Tbx3 and low miR-206 expression have a lower probability of survival when compared with patients with low Tbx3 and high miR-206 expression. These studies uncover a novel mechanism of Tbx3 regulation and identify a new target of the tumour suppressor miR-206. CONCLUSIONS The present study identified Tbx3 as a novel target of tumour suppressor miR-206 and characterised the miR-206/Tbx3 signalling pathway, which is involved in proliferation, invasion and maintenance of the cancer stem cell population in breast cancer cells. Our results suggest that restoration of miR-206 in Tbx3-positive breast cancer could be exploited for therapeutic benefit.
Nanomedicine (London, England) 2016 MAY
Evaluation of expansile nanoparticle tumor localization and efficacy in a cancer stem cell-derived model of pancreatic peritoneal carcinomatosis.
Herrera VL et al.
Abstract
AIM To evaluate the tumor localization and efficacy pH-responsive expansile nanoparticles (eNPs) as a drug delivery system for pancreatic peritoneal carcinomatosis (PPC) modeled in nude rats. METHODS & MATERIALS A Panc-1-cancer stem cell xeno1graft model of PPC was validated in vitro and in vivo. Tumor localization was tracked via in situ imaging of fluorescent eNPs. Survival of animals treated with paclitaxel-loaded eNPs (PTX-eNPs) was evaluated in vivo. RESULTS The Panc-1-cancer stem cell xenograft model recapitulates significant features of PPC. Rhodamine-labeled eNPs demonstrate tumor-specific, dose- and time-dependent localization to macro- and microscopic tumors following intraperitoneal injection. PTX-eNPs are as effective as free PTX in treating established PPC; but, PTX-eNPs result in fewer side effects. CONCLUSION eNPs are a promising tool for the detection and treatment of PPC.
Journal of cellular biochemistry 2016 JUN
Preservation of the 3D Phenotype Upon Dispersal of Cultured Cell Spheroids into Monolayer Cultures.
Koshkin V et al.
Abstract
In functional cytometric studies, cultured cells are exposed to effectors (e.g. drugs), and the heterogeneity of cell responses are studied using cytometry techniques (e.g. image cytometry). Such studies are difficult to perform on 3D cell cultures. A solution is to disperse 3D clusters and transfer the cells to the 2D state before applying effectors and using cytometry. This approach requires that the lifetime of the 3D phenotype be longer than the duration of the experiment. Here we studied the dynamics of phenotype transformation from 3D to 2D and searched for means of slowing this transformation down in dispersed spheroids of MCF7 cells. We found three functional biomarkers of the 3D phenotype in MCF7 cell spheroids that are absent in the 2D cell culture: (i) the presence of a subpopulation with an elevated drug-expelling capacity, (ii) the presence of a subpopulation with an elevated cytoprotective capacity and (iii) the accumulation of cells in the G1 phase of the cell cycle. Monitoring these biomarkers in cells transferred from the 3D state to the 2D state revealed their gradual extinction. We found that the combined application of an elevated cell density and thiol-containing medium supplements increased the lifetime of the 3D phenotype by several fold to as long as 96 h. Our results suggest that extending the lifetime of the 3D phenotype in the cells transferred from the 3D state to the 2D state can facilitate detailed functional cytometric studies, such as measurements of population heterogeneity of cytotoxicity, chemosensitivity and radiosensitivity. This article is protected by copyright. All rights reserved.
Biochemical and biophysical research communications 2016 JAN
Evodiamine selectively targets cancer stem-like cells through the p53-p21-Rb pathway.
Han S et al.
Abstract
In spite of the recent improvements, the resistance to chemotherapy/radiotherapy followed by relapse is the main hurdle for the successful treatment of breast cancer, a leading cause of death in women. A small population of breast cancer cells that have stem-like characteristics (cancer stem-like cells; CSLC) may contribute to this resistance and relapse. Here, we report on a component of a traditional Chinese medicine, evodiamine, which selectively targets CSLC of breast cancer cell lines MCF7 and MDAMB 231 at a concentration that does show a little or no cytotoxic effect on bulk cancer cells. While evodiamine caused the accumulation of bulk cancer cells at the G2/M phase, it did not hold CSLC in a specific cell cycle phase but instead, selectively killed CSLC. This was not due to the culture of CSLC in suspension or without FBS. A proteomic analysis and western blotting revealed that evodiamine changed the expression of cell cycle regulating molecules more efficiently in CSLC cells than in bulk cancer cells. Surprisingly, evodiamine selectively activated p53 and p21 and decreased inactive Rb, the master molecules in G1/S checkpoint. These data collectively suggest a novel mechanism involving CSLC-specific targeting by evodiamine and its possible use to the therapy of breast cancer.
Journal of cellular biochemistry 2016 JAN
Metabolic Suppression of a Drug-Resistant Subpopulation in Cancer Spheroid Cells.
Koshkin V et al.
Abstract
Inhibition of metabolic features which distinguish cancer cells from their non-malignant counterparts is a promising approach to cancer treatment. Energy support for drug extrusion in multidrug resistance (MDR) is a potential target for metabolic inhibition. Two major sources of ATP-based metabolic energy are partial (glycolysis) and complete (mitochondrial oxidative phosphorylation) oxidation of metabolic fuels. In cancer cells, the balance between them tends to be shifted toward glycolysis; this shift is considered to be characteristic of the cancer metabolic phenotype. Numerous earlier studies, conducted with cells cultured in a monolayer (2-D model), suggested inhibition of glycolytic ATP production as an efficient tool to suppress MDR in cancer cells. Yet, more recent work challenged the appropriateness of the 2-D model for such studies and suggested that a more clinically relevant approach would utilize a more advanced cellular model such as a 3-D model. Here, we show that the transition from the 2-D model (cultured monolayer) to a 3-D model (cultured spheroids) introduces essential changes into the concept of energetic suppression of MDR. The 3-D cell organization leads to the formation of a discrete cell subpopulation (not formed in the 2-D model) with elevated MDR transport capacity. This subpopulation has a specific metabolic phenotype (mixed glycolytic/oxidative MDR support) different from that of cells cultured in the 2-D model. Finally, the shift to the oxidative phenotype becomes greater when the spheroids are grown under conditions of lactic acidosis that are typical for solid tumors. The potential clinical significance of these findings is discussed.
View All Publications

 网站首页
网站首页