概要
Intestinal cultures generated using IntestiCult™ Organoid Differentiation Medium (Human) contain physiologically relevant proportions of differentiated and stem cell populations, recapitulating the diversity of the crypt-villus axis. When compared to conventional cell lines, intestinal monolayers exhibit greater barrier integrity, express higher levels of key differentiation markers, and have a morphology that is more representative of the in vivo intestine.
Applications of intestinal organoid cultures include studying the development and function of the intestinal epithelium, modeling intestinal diseases, compound screening, and regenerative therapy approaches. Intestinal monolayer and ALI cultures are particularly amenable for permeability assays and studies of infectious diseases due to easy access to the apical surface. This kit requires IntestiCult™ Organoid Growth Medium (Human; Catalog #06010) for the initiation and expansion of intestinal organoids prior to differentiation.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | IntestiCult™ | 100-0214 | All | English |
| Special Protocol 1 | IntestiCult™ Organoid Differentiation Medium (Human) | 100-0214 | All | English |
| Special Protocol 2 | IntestiCult™ Organoid Differentiation Medium (Human) | 100-0214 | All | English |
| Special Protocol 3 | IntestiCult™ Organoid Differentiation Medium (Human) | 100-0214 | All | English |
| Safety Data Sheet 1 | IntestiCult™ | 100-0214 | All | English |
| Safety Data Sheet 2 | IntestiCult™ | 100-0214 | All | English |
数据及文献
Data
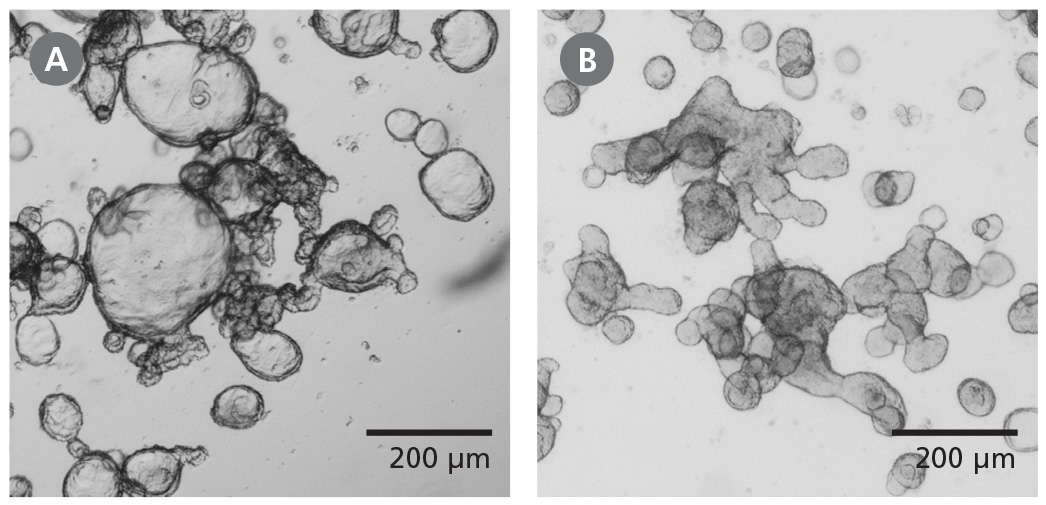
Figure 1. Differentiated Human Intestinal Organoids Display a Budded Morphology
(A) Organoids grown in IntestiCult™ OGM are primarily cystic. (B) When switched to IntestiCult™ ODM, organoids develop a thickened epithelium with a pronounced, budded morphology indicative of a more differentiated state. Organoids were imaged on day 5 of expansion or differentiation respectively.
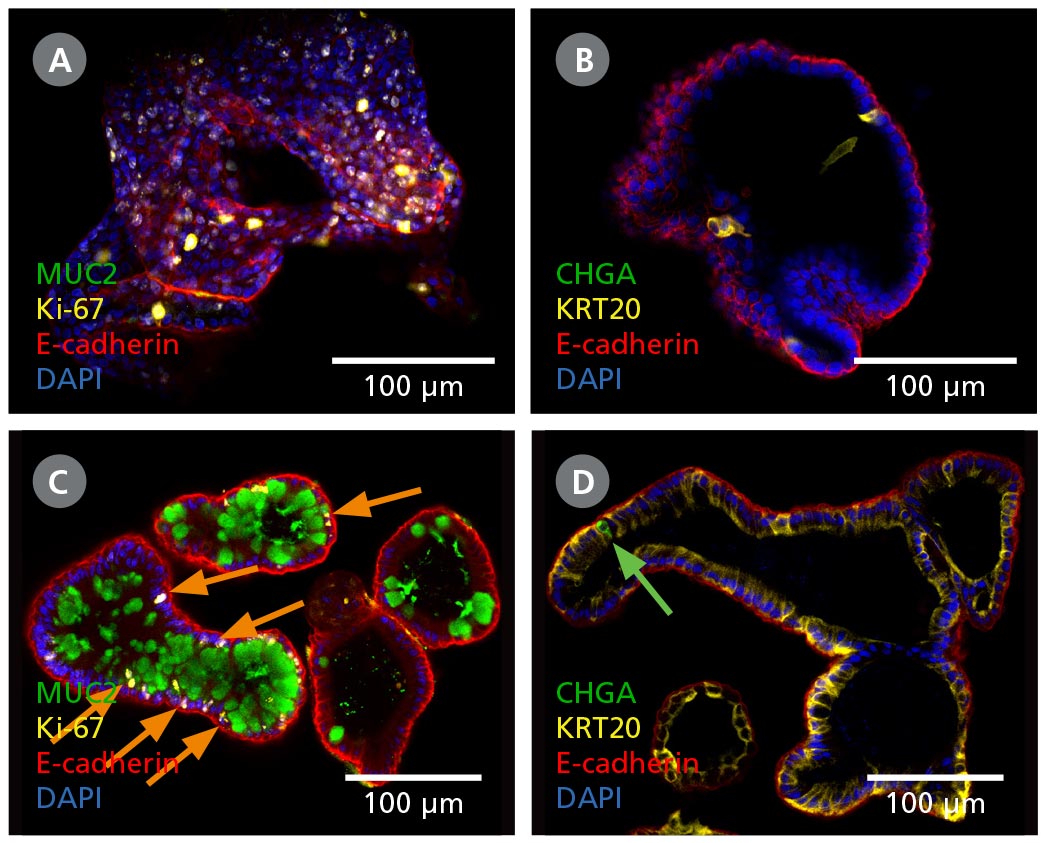
Figure 2. Intestinal Organoids Contain a Higher Proportion of Mature Cell Types Following Differentiation in IntestiCult™ ODM
(A, B) Organoids grown in IntestiCult™ OGM are enriched for Ki-67+ proliferative cells (A), while containing few differentiated cell types such as goblet cells (MUC2, A), enterocytes (KRT20, B), and enteroendocrine cells (CHGA, B). (C, D) When switched to IntestiCult™ ODM, organoids contain a small number of Ki-67+ proliferative cells (C, arrows), with more physiological proportions of goblet cells (MUC2, C), enterocytes (KRT20, D), and chromogranin A- (CHGA-)positive enteroendocrine cells (D, arrow).
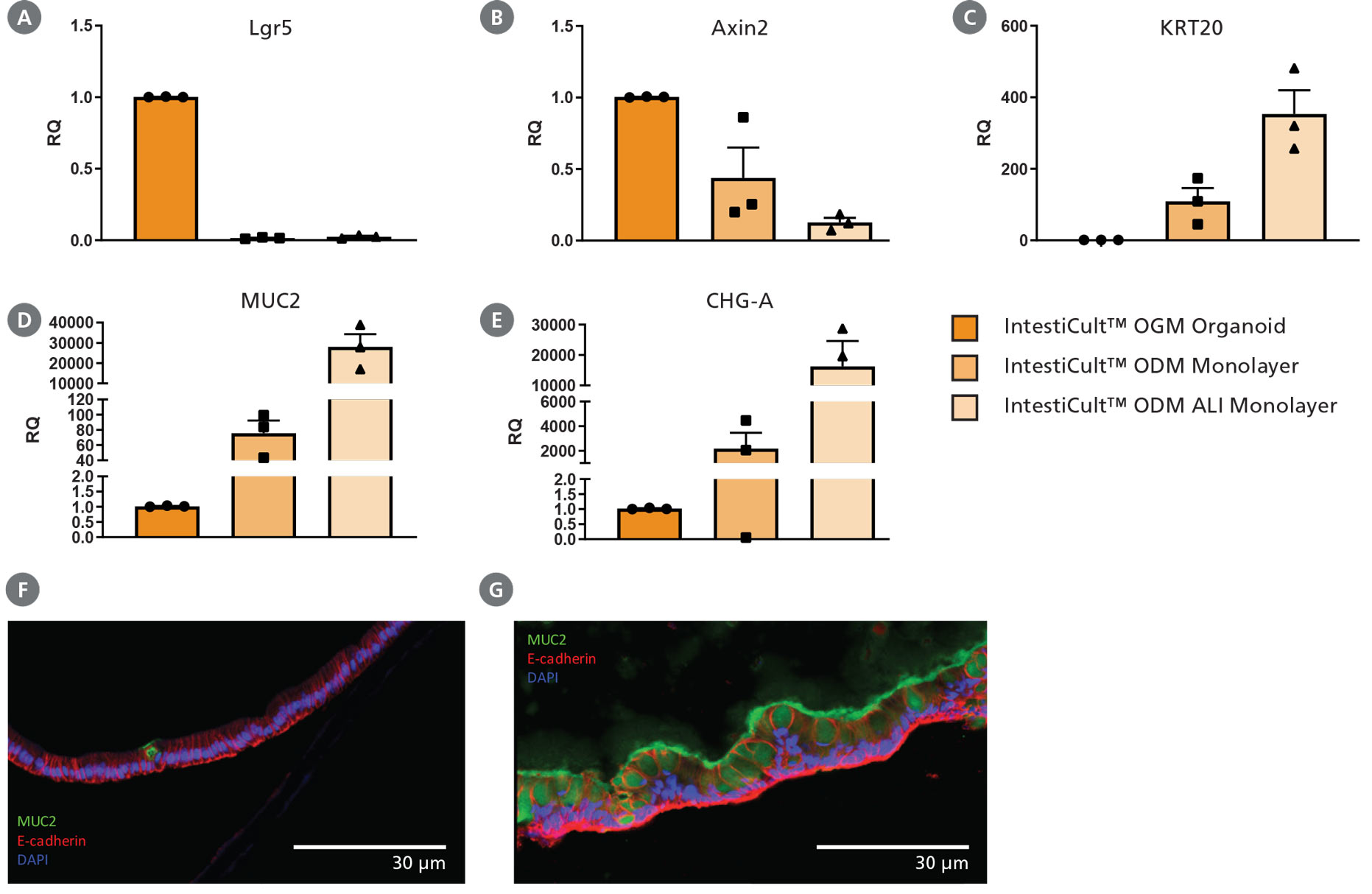
Figure 3. Differentiation of Intestinal Epithelium at the Air-Liquid Interface (ALI) Using IntestiCult™ ODM
(A – E) Growing organoid-derived monolayers as ALI cultures drives further differentiation of intestinal epithelial cultures as seen by changes in gene expression measured by RT-qPCR. Relative quantification (RQ) for each marker is shown relative to actB and TBP housekeeping genes and normalized with respect to undifferentiated organoids grown in IntestiCult OGM (Human). Progenitor markers (A) Lgr5 and (B) Axin2 are significantly reduced in both submerged monolayers and ALI cultures, while markers of enterocytes (KRT20, C), goblet cells (MUC2, D), and enteroendocrine cells (CHGA, E) are significantly increased. Further reduction in Axin2 is seen in ALI monolayers with an increase in expression of KRT20, MUC2, and CHGA. (F, G) Comparing cross-sections of organoid monolayers grown in IntestiCult™ ODM as (F) submerged culture or (G) at the ALI shows further differentiation of the intestinal epithelium with an increased proportion of goblet cells and extracellular mucus (MUC2, green).
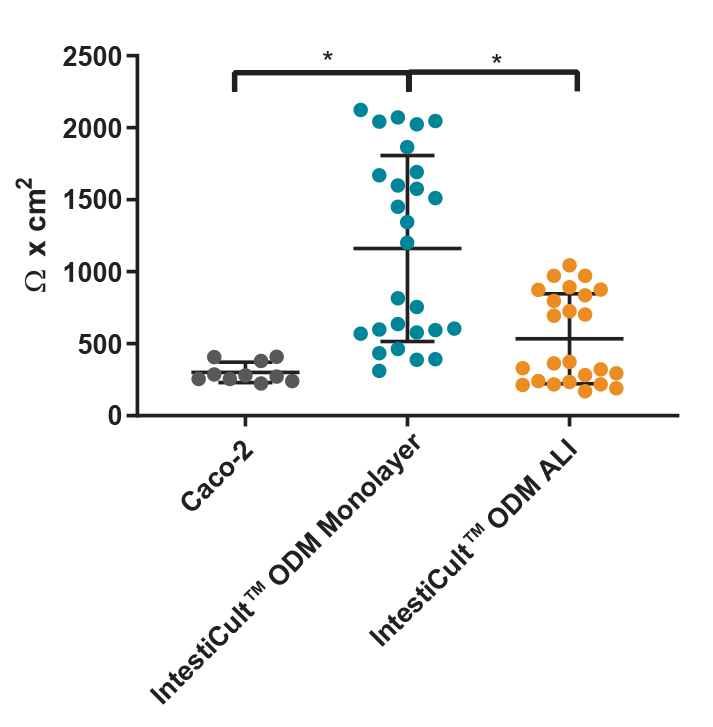
Figure 4. Differentiated Organoid-Derived Monolayers and ALI Cultures Display More Physiological Trans-Epithelial Electrical Resistance (TEER) than Caco-2 Cells
Differentiated organoid-derived monolayers grown as a submerged monolayer (IntestiCult™ ODM Monolayer), or at the ALI (IntestiCult™ ODM ALI), show higher TEER values as compared to Caco-2 cultures.Organoid-derived monolayers grown at the ALI show a loosening of tight junctions due to further differentiation of the brush border, and thus lower TEER values are observed. * p < 0.0001.
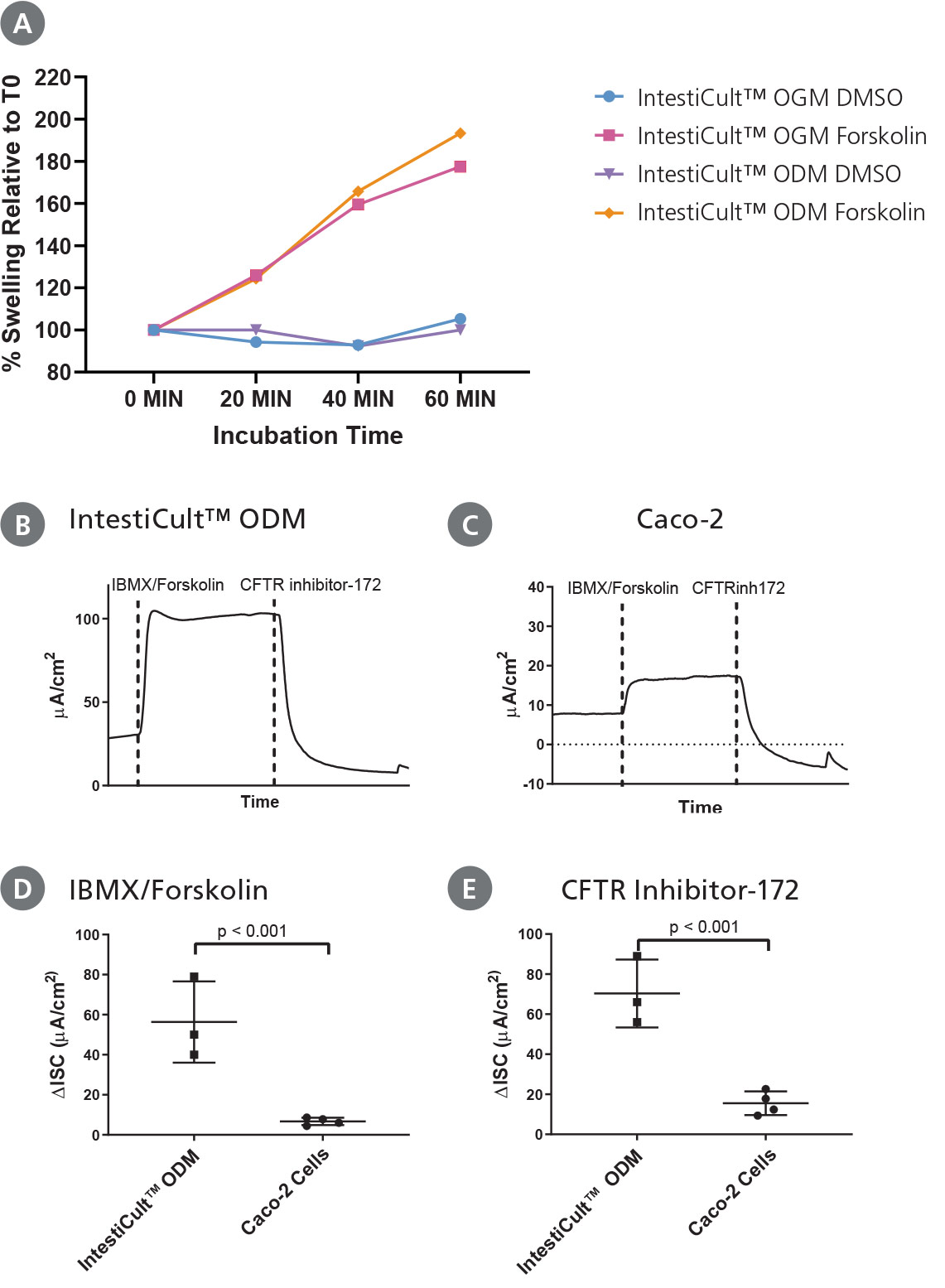
Figure 5. Differentiated Intestinal Organoids Provide a Suitable Model for Studying CFTR Response In Vitro
(A) Organoids differentiated further in IntestiCult™ ODM show a comparable degree of swelling when treated with forskolin as compared to organoids grown in IntestiCult™ OGM, demonstrating suitability for use in forskolin-induced swelling assays. (B – E) Ussing chamber analysis of submerged (B) organoid-derived monolayers and (C) Caco-2 cultures demonstrate increased sensitivity of organoid-derived monolayers to CFTR activation and inhibition by IBMX/Forskolin and CFTR Inhibitor-172 respectively. (D, E) Analysis of CFTR modulation by IBMX/Forskolin and CFTR Inhibitor-172 show significantly greater (D) activation and (E) inhibition of CFTR activity in organoid-derived monolayers as compared to Caco-2 cultures (p < 0.001 for both).

 网站首页
网站首页