概要
The ImmunoCult™ Human CD3/CD28 T Cell Activator can be used on the Seahorse XF Analyzer to measure T cell activation response and is also available as part of the Agilent Seahorse XF Hu T Cell Activation Assay Kit.
This product is designed for cell therapy research applications following the recommendations of USP<1043> on Ancillary Materials, and we can currently work with you to qualify this reagent under an approved investigational new drug (IND) or clinical trial application (CTA).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | ImmunoCult™ Human CD3/CD28 T Cell Activator | 10971, 10991 | All | English |
| Safety Data Sheet | ImmunoCult™ Human CD3/CD28 T Cell Activator | 10971, 10991 | All | English |
数据及文献
Data
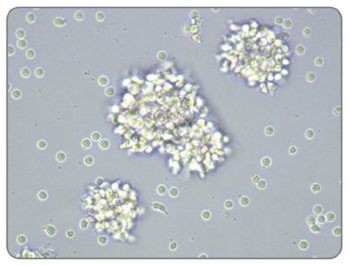
Figure 1. Activated Morphology of Human T Cells Stimulated With ImmunoCult™ Human CD3/CD28 T Cell Activator
Image of human T cells isolated using the EasySep™ Human T Cell Isolation Kit (Catalog #17951), stimulated with ImmunoCult™ Human CD3/CD28 T Cell Activator, and cultured in ImmunoCult™-XF T Cell Expansion Medium (Catalog # 10981).
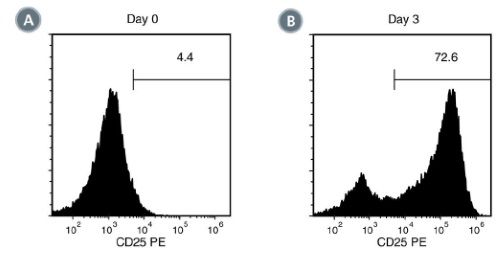
Figure 2. Activation of EasySep™-isolated Human T Cells stimulated With ImmunoCult™ Human CD3/CD28 T Cell Activator
EasySep™-isolated human T cells were stimulated with ImmunoCult™ Human CD3/CD28 T Cell Activator and cultured in ImmunoCult™-XF T Cell Expansion Medium. Activation of viable CD3+ T cells was assessed by CD25 expression using flow cytometry. On day 0, the frequency of CD25 positive cells was (A) 5.6 ± 2.4% (mean ± SD). Following 3 days of culture, the frequency of CD25 positive cells was (B) 75.4 ± 13.8% (mean ± SD) when stimulated with ImmunoCult™ Human CD3/CD28 T Cell Activator.
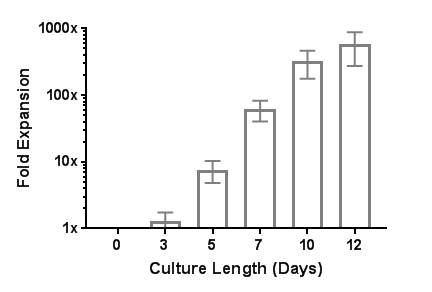
Figure 3. Robust Human T Cell Expansion with ImmunoCult™ Human CD3/CD28 T Cell Activator
EasySep™-isolated human T cells were expanded over 12 days with ImmunoCult™ Human CD3/CD28 T Cell Activator in ImmunoCult™-XF T Cell Expansion Medium supplemented with Human Recombinant IL-2. On day 0, 1 x 10^6 EasySep™-isolated human T cells were stimulated with 25 μL of ImmunoCult™ Human CD3/CD28 T Cell Activator in ImmunoCult™-XF T Cell Expansion Medium supplemented with 10 ng/mL Human Recombinant IL-2. On days 3, 5, 7, and 10, viable cells were counted and fresh medium supplemented with IL-2 was added. No additional ImmunoCult™ Human CD3/CD28 T Cell Activator was added during the 12-day culture period (mean ± SD in 6 experiments with 3 donors).

 网站首页
网站首页