概要
ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator is designed to activate and expand human T cells in the absence of magnetic beads, feeder cells or antigen. ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator consists of soluble tetrameric antibody complexes that bind CD3, CD28 and CD2 cell surface ligands. Binding of the tetrameric antibody complexes results in the cross-linking of CD3, CD28, and CD2 cell surface ligands, thereby providing the required primary and co-stimulatory signals for T cell activation. Activated T cells can be expanded in ImmunoCult™-XF T Cell Expansion Medium or other media for culturing human T cells supplemented with cytokines.
This product is designed for cell therapy research applications following the recommendations of USP<1043> on Ancillary Materials, and we can currently work with you to qualify this reagent under an approved investigational new drug (IND) or clinical trial application (CTA).
数据及文献
Publications (6)
Cancer research 2020 sep
Plasma Gelsolin Inhibits CD8+ T-cell Function and Regulates Glutathione Production to Confer Chemoresistance in Ovarian Cancer.
M. Asare-Werehene et al.
Abstract
Although initial treatment of ovarian cancer is successful, tumors typically relapse and become resistant to treatment. Because of poor infiltration of effector T cells, patients are mostly unresponsive to immunotherapy. Plasma gelsolin (pGSN) is transported by exosomes (small extracellular vesicle, sEV) and plays a key role in ovarian cancer chemoresistance, yet little is known about its role in immunosurveillance. Here, we report the immunomodulatory roles of sEV-pGSN in ovarian cancer chemoresistance. In chemosensitive conditions, secretion of sEV-pGSN was low, allowing for optimal CD8+ T-cell function. This resulted in increased T-cell secretion of IFN$\gamma$, which reduced intracellular glutathione (GSH) production and sensitized chemosensitive cells to cis-diaminedichloroplatinum (CDDP)-induced apoptosis. In chemoresistant conditions, increased secretion of sEV-pGSN by ovarian cancer cells induced apoptosis in CD8+ T cells. IFN$\gamma$ secretion was therefore reduced, resulting in high GSH production and resistance to CDDP-induced death in ovarian cancer cells. These findings support our hypothesis that sEV-pGSN attenuates immunosurveillance and regulates GSH biosynthesis, a phenomenon that contributes to chemoresistance in ovarian cancer. SIGNIFICANCE: These findings provide new insight into pGSN-mediated immune cell dysfunction in ovarian cancer chemoresistance and demonstrate how this dysfunction can be exploited to enhance immunotherapy.
Science advances 2020 may
Competition between PAF1 and MLL1/COMPASS confers the opposing function of LEDGF/p75 in HIV latency and proviral reactivation.
R. Gao et al.
Abstract
Transcriptional status determines the HIV replicative state in infected patients. However, the transcriptional mechanisms for proviral replication control remain unclear. In this study, we show that, apart from its function in HIV integration, LEDGF/p75 differentially regulates HIV transcription in latency and proviral reactivation. During latency, LEDGF/p75 suppresses proviral transcription via promoter-proximal pausing of RNA polymerase II (Pol II) by recruiting PAF1 complex to the provirus. Following latency reversal, MLL1 complex competitively displaces PAF1 from the provirus through casein kinase II (CKII)-dependent association with LEDGF/p75. Depleting or pharmacologically inhibiting CKII prevents PAF1 dissociation and abrogates the recruitment of both MLL1 and Super Elongation Complex (SEC) to the provirus, thereby impairing transcriptional reactivation for latency reversal. These findings, therefore, provide a mechanistic understanding of how LEDGF/p75 coordinates its distinct regulatory functions at different stages of the post-integrated HIV life cycles. Targeting these mechanisms may have a therapeutic potential to eradicate HIV infection.
Stem Cell Research 2019 oct
Detection of all adult Tau isoforms in a 3D culture model of iPSC-derived neurons
L. Miguel et al.
Abstract
Tauopathies are a class of neurodegenerative diseases characterized by the presence of pathological intracellular deposits of Tau proteins. Six isoforms of Tau are expressed in the adult human brain, resulting from alternative splicing of the MAPT gene. Tau splicing is developmentally regulated such that only the smallest Tau isoform is expressed in fetal brain, contrary to the adult brain showing the expression of all 6 isoforms. Induced Pluripotent Stem Cell (iPSC) technology has opened up new perspectives in human disease modeling, including tauopathies. However, a major challenge to in vitro recapitulation of Tau pathology in iPSC-derived neurons is their relative immaturity. In this study, we examined the switch in Tau splicing from fetal-only to all adult Tau isoforms during the differentiation of iPSC-derived neurons in a new 3D culture system. First, we showed that iPSC-induced neurons inside Matrigel-coated alginate capsules were able to differentiate into cortical neurons. Then, using a new assay that allowed both the qualitative and the quantitative analysis of all adult MAPT mRNA isoforms individually, we demonstrated that BrainPhys-maintained neurons expressed the 6 adult MAPT mRNA transcripts from 25 weeks of maturation, making this model highly suitable for modeling Tau pathology and therapeutic purposes.
Scientific reports 2019 nov
PD-1+ melanocortin receptor dependent-Treg cells prevent autoimmune disease.
F. Muhammad et al.
Abstract
Experimental autoimmune uveoretinitis (EAU) is a mouse model of human autoimmune uveitis marked by ocular autoantigen-specific regulatory immunity in the spleen. The melanocortin 5 receptor (MC5r) and adenosine 2 A receptor (A2Ar) are required for induction of post-EAU regulatory T cells (Tregs) which provide resistance to EAU. We show that blocking the PD-1/PD-L1 pathway prevented suppression of EAU by post-EAU Tregs. A2Ar induction of PD-1+FoxP3+ Tregs in uveitis patients was similar compared to healthy controls, but was significantly reduced with melanocortin stimulation. Further, lower body mass index correlated with responsiveness to stimulation of this pathway. These observations indicate an importance of the PD-1/PD-L1 pathway to provide resistance to relapsing uveitis and shows a reduced capacity of uveitis patients to induce Tregs when stimulated through melanocortin receptors, but that it is possible to bypass this part of the pathway through direct stimulation of A2Ar.
The Journal of clinical investigation 2019 dec
Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations.
A. H. Mandarano et al.
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease with no known cause or mechanism. There is an increasing appreciation for the role of immune and metabolic dysfunction in the disease. ME/CFS has historically presented in outbreaks, often has a flu-like onset, and results in inflammatory symptoms. Patients suffer from severe fatigue and post-exertional malaise. There is little known about the metabolism of specific immune cells in ME/CFS patients. To investigate immune metabolism in ME/CFS, we isolated CD4+ and CD8+ T cells from 53 ME/CFS patients and 45 healthy controls. We analyzed glycolysis and mitochondrial respiration in resting and activated T cells, along with markers related to cellular metabolism, and plasma cytokines. We found that ME/CFS CD8+ T cells have reduced mitochondrial membrane potential compared to healthy controls. Both CD4+ and CD8+ T cells from ME/CFS patients had reduced glycolysis at rest, while CD8+ T cells also had reduced glycolysis following activation. ME/CFS patients had significant correlations between measures of T cell metabolism and plasma cytokine abundance that differed from healthy control subjects. Our data indicate that patients have impaired T cell metabolism consistent with ongoing immune alterations in ME/CFS that may illuminate the mechanism behind this disease.
Blood 2016 JUL
LFA-1 integrin antibodies inhibit leukocyte α4β1-mediated adhesion by intracellular signaling.
Grö et al.
Abstract
Binding of ICAM-1 (intercellular adhesion molecule-1) to the β2-integrin LFA-1 (leukocyte function associated antigen-1) is known to induce crosstalk to the α4β1 integrin. Using different LFA-1 monoclonal antibodies we have been able to study the requirement and mechanism of action for the crosstalk in considerable detail. LFA-1 activating antibodies and those inhibitory antibodies that signal to α4β1 induce phosphorylation of Thr-758 on the β2-chain, which is followed by binding of 14-3-3 proteins and signaling through the G protein exchange factor Tiam1. This results in dephosphorylation of Thr-788/789 on the β1-chain of α4β1 and loss of binding to its ligand VCAM-1 (vascular cell adhesion molecule-1). The results show that with LFA-1 antibodies, we can either 1) activate LFA-1 and inhibit α4β1, 2) inhibit both LFA-1 and α4β1, 3) inhibit LFA-1 but not α4β1 or 4) not affect LFA-1 or α4β1 These findings are important for the understanding of integrin regulation and for the interpretation of the effect of integrin antibodies and their use in clinical applications.
View All Publications

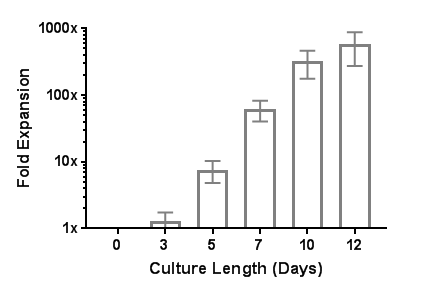

 网站首页
网站首页