概要
Applications of BrainPhys™ Neuronal Medium include culture of primary neurons, differentiation and maturation of hPSC-derived neurons, microelectrode array-based recording of neuronal activity, live fluorescent imaging (including calcium imaging and optogenetics) and transdifferentiation of somatic cells to neurons.
To ensure cell survival in long-term serum-free culture, BrainPhys™ must be combined with an appropriate supplement. For your convenience, various BrainPhys™ kits are available for primary or hPSC-derived neurons. BrainPhys™ Neuronal Medium and SM1 Kit (Catalog #05792) is recommended for culture of primary neurons. BrainPhys™ Primary Neuron Kit (Catalog #05794) is recommended for plating and culture of primary neurons. BrainPhys™ Neuronal Medium N2-A & SM1 Kit (Catalog #05793) and BrainPhys™ hPSC Neuron Kit (Catalog #05795) are recommended for the differentiation and maturation of hPSC-derived neurons, in combination with lineage-specific growth factors and/or small molecules (if necessary).
技术资料
数据及文献
Data
Table 1. Properties of Culture Media (C Bardy et al. Proc Natl Acad Sci USA, 2015)
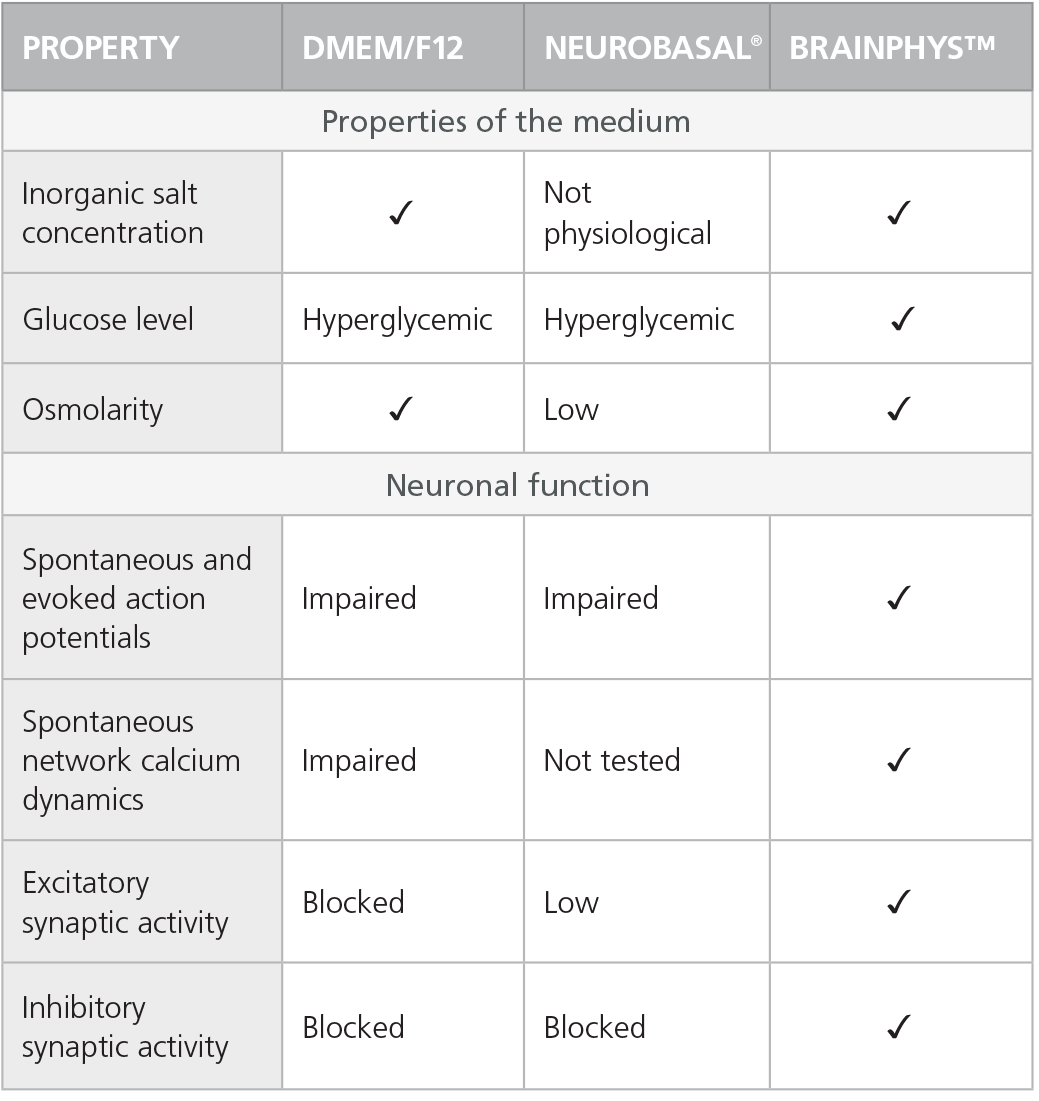
Check-mark denotes physiological conditions and supported activities according to C Bardy et al. Proc Natl Acad Sci USA, 2015.
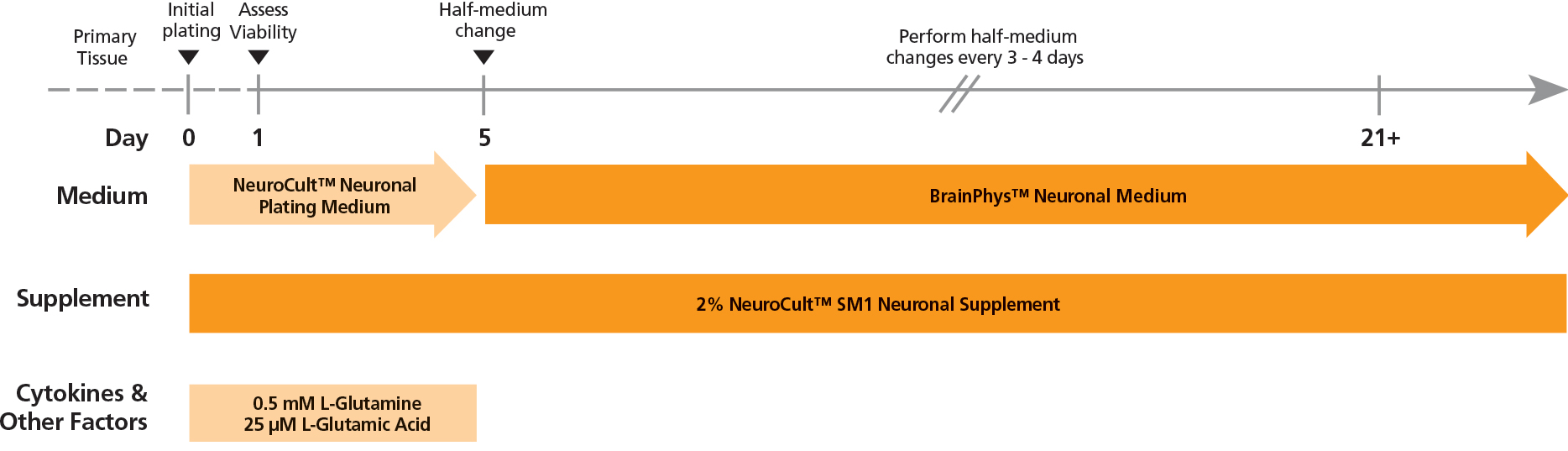
Figure 1. Protocol for Plating and Culturing Primary Neurons with the SM1 Culture System
Primary rodent tissue dissociated in papain was plated in NeuroCult™ Neuronal Plating Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, L-Glutamine, and L-Glutamic Acid. On day 5, primary neurons were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, by performing half-medium changes every 3 - 4 days.
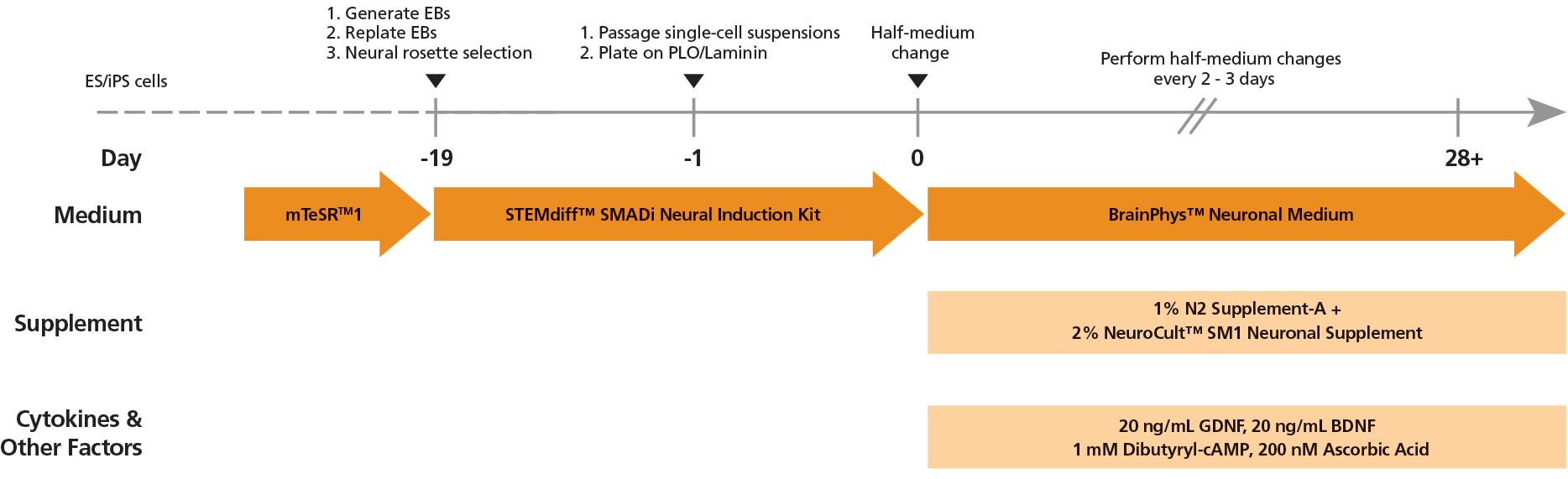
Figure 2. Protocol for Culturing hPSCs with the SM1 Culture System
hPSCs were maintained in mTeSR™1 medium and then differentiated using the STEMdiff™ SMADi Neural Induction Kit. Following plating on PLO/laminin, half-medium changes were performed to transition to BrainPhys™ Neuronal Medium for maturation and long-term culture.
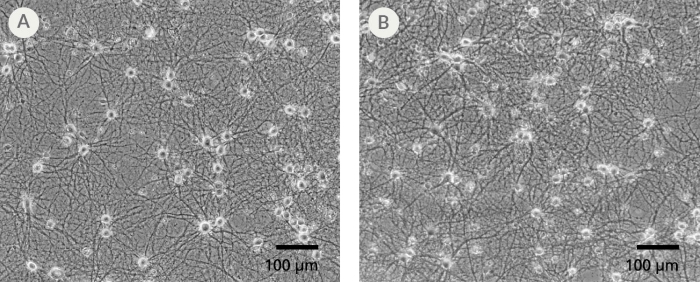
Figure 3. The SM1 Culture System Supports Long-Term Culture of Rodent Neurons
Primary E18 rat cortical neurons were cultured in the SM1 Culture System. A large number of viable neurons are visible after (A) 21 and (B) 35 days, as demonstrated by their bright neuronal cell bodies, and extensive neurite outgrowth and branching. Neurons are evenly distributed over the culture surface with minimal cell clumping.
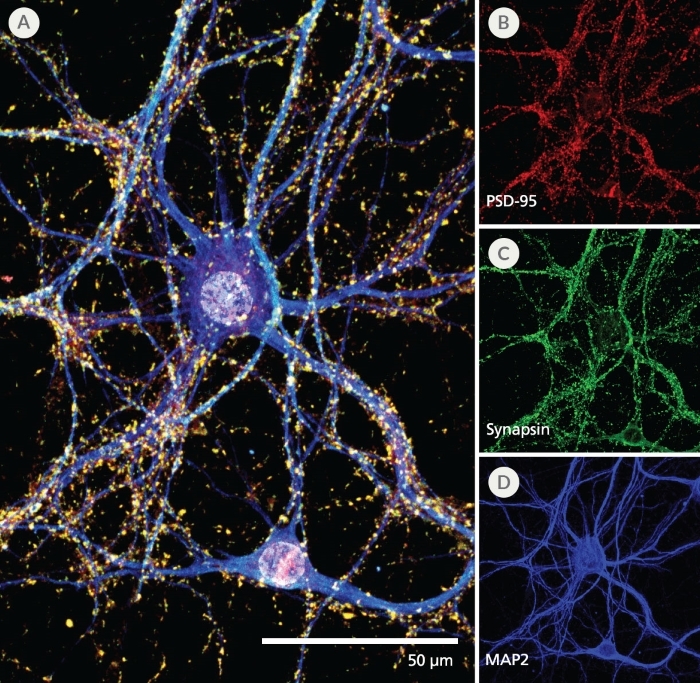
Figure 4. Pre- and Post-Synaptic Markers are Expressed in Rodent Neurons Cultured in the SM1 Culture System
Primary E18 rat cortical neurons were cultured in the SM1 Culture System. At 21 DIV, neurons are phenotypically mature, as indicated by the presence of an extensive dendritic arbor, and appropriate expression and localization of pre-synaptic synapsin (A,C; green) and post-synaptic PSD-95 (A,B; red) markers. Synapsin is concentrated in discrete puncta distributed along the somata and dendritic processes, as defined by the dendritic marker MAP2 (A,D; blue).
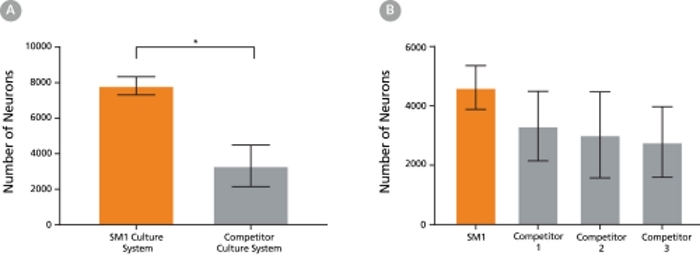
Figure 5. The SM1 Culture System Supports Increased Cell Survival
(A) Primary E18 rat cortical neurons were cultured in the SM1 Culture System or a Competitor Culture System for 21 days. Neurons cultured in the SM1 Culture System have a significantly higher number of viable cells compared to the competitor culture system (n = 4; mean ± 95% CI; *p < 0.05). (B) Primary E18 rat cortical neurons were cultured in Neurobasal® supplemented with NeuroCult™ SM1 Neuronal Supplement (SM1) or competitor B27-like supplements (Competitor 1,2,3) for 21 days. Cultures supplemented with NeuroCult™ SM1 Neuronal Supplement have an equal number of neurons compared to competitor-supplemented cultures. Bars represent standard error of mean.
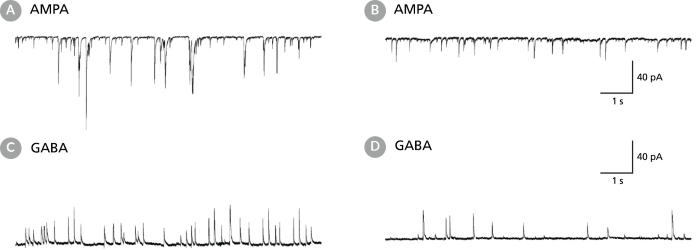
Figure 6. Rodent Neuronal Cultures Matured in BrainPhys™ Neuronal Medium Show Improved Excitatory and Inhibitory Synaptic Activity
(A,C) Primary rat E18 cortical neurons were plated in NeuroCult™ Neuronal Basal Medium (product superseded with NeuroCult™ Neuronal Plating Medium which is a part of the BrainPhys™ Primary Neuron Kit), supplemented with NeuroCult™ SM1 Neuronal Supplement. After 5 DIV, the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, by performing half-medium changes every 3 - 4 days. Neurons were cultured for 21 DIV. (B,D) Primary rat E18 cortical neurons were plated and matured in a competitor neuronal medium (Neurobasal®), supplemented with NeuroCult™ SM1 Neuronal Supplement for 21 DIV. (A,C) Neurons matured in BrainPhys™ Neuronal Medium showed spontaneous excitatory (AMPA-mediated; A) and inhibitory (GABA-mediated; C) synaptic events. The frequency and amplitude of spontaneous synaptic events is consistently greater in neuronal cultures matured in BrainPhys™ Neuronal Medium, compared to neurons plated and matured in a competitor neuronal medium (B,D). Traces are representative.
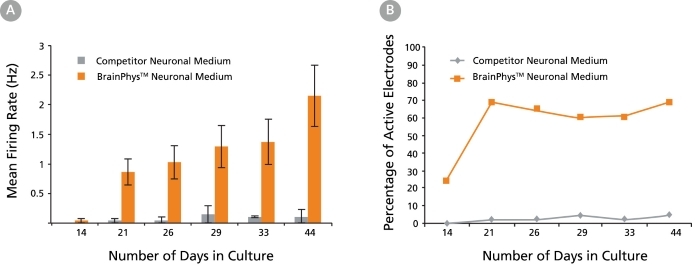
Figure 7. Primary Neuronal Cultures Matured in BrainPhys™ Neuronal Medium Show Improved Electrical Activity in Microelectrode-Array Systems
Primary rat E18 cortical neurons were plated in a competitor neuronal medium (Neurobasal®) supplemented with NeuroCult™ SM1 Neuronal Supplement. After 5 DIV, half of the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, by performing half-medium changes every 3 - 4 days. The other half of the cultures were maintained in the competitor neuronal medium throughout. The electrical activities of the neuronal cultures were measured twice a week using a microelectrode array (MEA) system (Axion Biosystems). (A) The mean firing rate of neurons cultured in BrainPhys™ Neuronal Medium increases over time, whereas the mean firing rate of neurons in the competitor neuronal medium condition remains low (n = 1; mean ± SEM, 128 electrodes). (B) The percentage of active electrodes (>0.005 Hz) of neurons matured in BrainPhys™ Neuronal Medium increases from 24% on day 14 to 69% on day 21, and then remains stable at 60 – 70% from days 21 – 44. In contrast, < 5% of electrodes was active in the competitor neuronal medium condition over the same 6-week period.
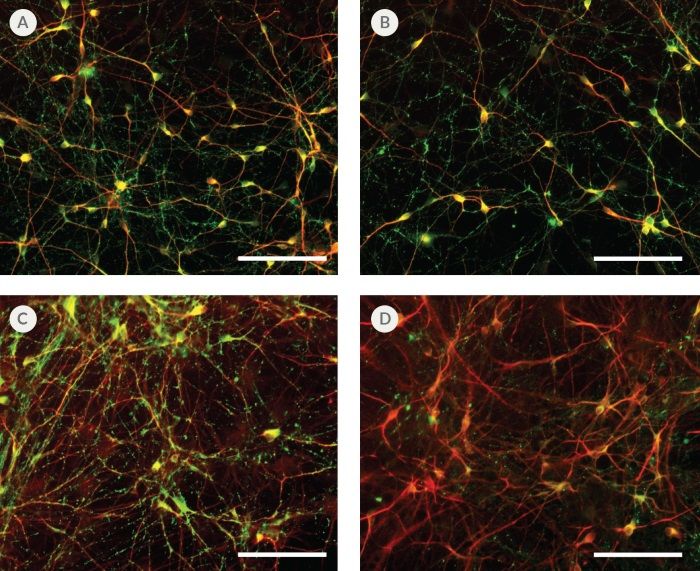
Figure 8. hPSC-Derived Neurons Generated in BrainPhys™ Neuronal Medium Express Markers of Neuronal Maturity After 14 and 44 Days of Differentiation
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured in (A,C) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B,D) DMEM/F12 under the same supplementation conditions. After 14 and 44 days of differentiation and maturation, neurons express the synaptic marker Synapsin 1 (green) and the mature neuronal marker MAP2 (red). In this example, neurons matured in BrainPhys™ Neuronal Medium show increased Synapsin 1 staining. Scale bar= 100 µm
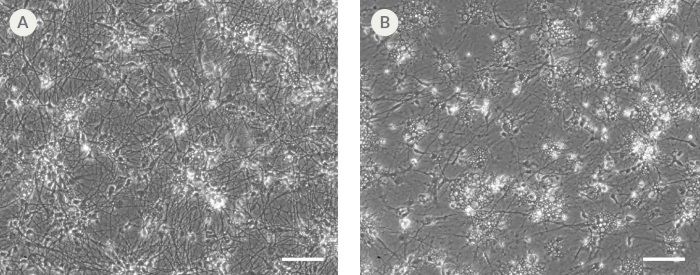
Figure 9. hPSC-Derived Neurons Generated in BrainPhys™ Neuronal Medium and NeuroCult™ SM1 and N2 Supplements are Healthy and Morphologically Normal
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured for 44 DIV in (A) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B) DMEM/F12 under the same supplementation conditions. Neuronal cultures differentiated from NPCs in BrainPhys™ Neuronal Medium display extensive neurite outgrowth and reduced cellular debris compared to cultures differentiated in DMEM/F12. Scale bar= 100 µm.
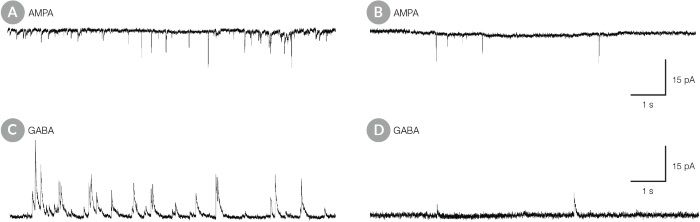
Figure 10. hPSC-Derived Neurons Matured in BrainPhys™ Neuronal Medium Show Improved Excitatory and Inhibitory Synaptic Activity
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured for 44 DIV in (A,C) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B,D) in DMEM/F12 under the same supplementation conditions. (A,C) Neurons matured in BrainPhys™ Neuronal Medium showed spontaneous excitatory (AMPA-mediated; A) and inhibitory (GABA-mediated; C) synaptic events. The frequency and amplitude of spontaneous synaptic events is consistently greater in neuronal cultures matured in BrainPhys™ Neuronal Medium, compared to neurons plated and matured in DMEM/F12 (B,D). Traces are representative.

 网站首页
网站首页