概要
HemaTox™ Myeloid Kit includes a specialized serum-free culture medium and 100X supplement. Complete HemaTox™ Myeloid Medium (HemaTox™ Myeloid Medium + HemaTox™ Myeloid 100X Supplement) promotes the proliferation of human CD34+ HSPCs and their differentiation into myeloid cells during a 7-day culture period. After culture, the cells can be counted and assessed for expression of myeloid markers such as CD13, CD14, and CD15 using flow cytometry or other methods.
HemaTox™ Myeloid Kit may be used on its own or in combination with HemaTox™ Erythroid Kit (Catalog #09701) or HemaTox™ Megakaryocyte Kit (Catalog #09707) to assess lineage-specific drug toxicity in parallel.
Each kit contains sufficient medium and supplement for testing up to 160 different conditions (triplicate wells per condition, 200 µL per well) in 5 x 96-well plates.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | HemaTox™ Myeloid Kit | 09704 | All | English |
| Safety Data Sheet 1 | HemaTox™ Myeloid Kit | 09704 | All | English |
| Safety Data Sheet 2 | HemaTox™ Myeloid Kit | 09704 | All | English |
数据及文献
Data

Figure 1. General HemaTox™ Myeloid Kit Procedure
*The cell isolation step may be omitted if pre-enriched CD34+ cells are used.

Figure 2. Flow Cytometry Plot Showing Cells Produced After Culture of CD34+ HSPCs with the HemaTox™ Myeloid Kit
(A) Human CB CD34+ cells were cultured with the HemaTox™ Myeloid Kit using the protocol as written in the Product Information Sheet (PIS). (B) After the appropriate culture period, cells were harvested and stained for cell surface proteins expressed on myeloid (CD13 and CD15) cells.

Figure 3. Reproducibility of HemaTox™ Myeloid Kit Results Between Experiments and Using Different CD34+ Cell Preparations
(A) Dose-response curves were generated from titrations of 5-FU added to human CB CD34+ cells isolated from 3 donors and cultured with the HemaTox™ Myeloid Kit. Three to five separate experiments were performed with cells from each donor. In each assay similar IC50 values were obtained with cells from different donors and in different experiments with cells from the same donor. Shown are values normalized to the percentages (%) of maximum cell growth without drug. Despite differences in absolute cell counts, curves are reproducible when normalized within each experiment. (B) Table showing IC50 values generated for 5-FU in culture with the myeloid kit including standard deviation (SD) and the coefficient of variation (% CV) across experiments.

Figure 4. Correlation Between IC50 Values for Six Drugs Measured Using the CFU-GM Assay and the 96-Well Plate Liquid Culture-Based HemaTox™ Myeloid Kit
Human BM CD34+ cells were cultured in colony-forming unit - granulocyte/macrophage (CFU-GM) assays with MethoCult™ medium and in liquid culture with the HemaTox™ Myeloid Kit. IC50 values generated using each assay were plotted on the X and Y axes and shown to be highly correlated with a coefficient of determination (R2) of 0.91.
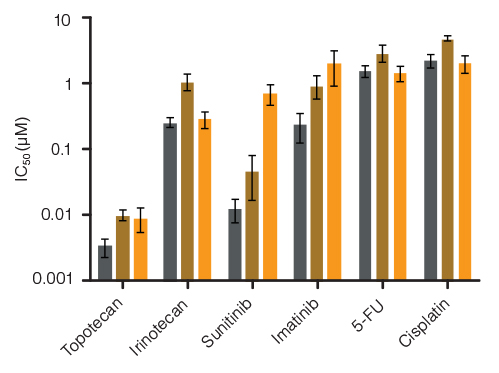
Figure 5. Lineage-Specific Differences in Hematotoxicity Identified with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
Results show average IC50 values for each drug tested on human BM CD34+ cells using the HemaTox™ Erythroid (grey), Myeloid (gold) and Megakaryocyte (orange) Kits. Most drugs show similar toxicity for each lineage but some, such as Sunitinib, are ~100-fold more toxic for erythroid than for megakaryocyte progenitor differentiation with intermediate toxicity for myeloid progenitor differentiation. Vertical lines indicate standard error of the mean (SEM) (n = 4 - 8).

 网站首页
网站首页