概要
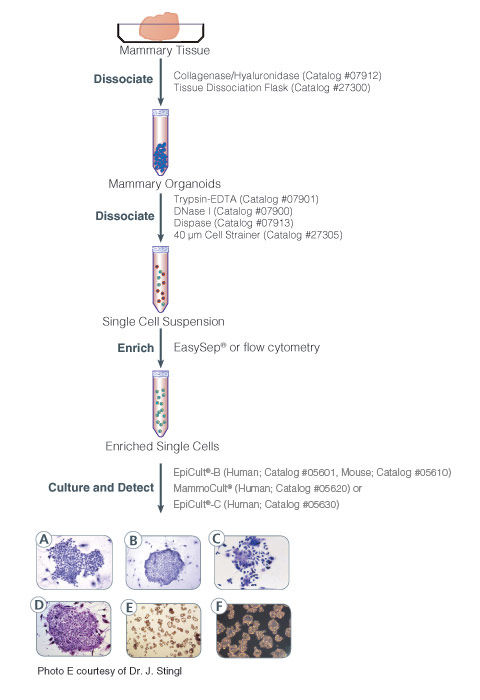
EpiCult™-B (mouse) is a serum-free liquid culture medium optimized for the culture of mouse mammary luminal and myoepithelial cells. It is ideal for the culture and evaluation of mouse mammary epithelial progenitors in the mammary colony-forming unit assay when used in conjunction with an irradiated feeder layer such as NIH 3T3 cells. This medium is also used for enzymatic dissociation of mouse mammary tissue when supplemented with collagenase and hyaluronidase. Addition of Human Recombinant EGF (Catalog #78006), Human Recombinant bFGF (Catalog #78003), and Heparin Solution (Catalog #07980) is required for culturing cells.
数据及文献
Publications (9)
Nature communications 2019
Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism.
R. Bertolio et al.
Abstract
Sterol regulatory element binding proteins (SREBPs) are a family of transcription factors that regulate lipid biosynthesis and adipogenesis by controlling the expression of several enzymes required for cholesterol, fatty acid, triacylglycerol and phospholipid synthesis. In vertebrates, SREBP activation is mainly controlled by a complex and well-characterized feedback mechanism mediated by cholesterol, a crucial bio-product of the SREBP-activated mevalonate pathway. In this work, we identified acto-myosin contractility and mechanical forces imposed by the extracellular matrix (ECM) as SREBP1 regulators. SREBP1 control by mechanical cues depends on geranylgeranyl pyrophosphate, another key bio-product of the mevalonate pathway, and impacts on stem cell fate in mouse and on fat storage in Drosophila. Mechanistically, we show that activation of AMP-activated protein kinase (AMPK) by ECM stiffening and geranylgeranylated RhoA-dependent acto-myosin contraction inhibits SREBP1 activation. Our results unveil an unpredicted and evolutionary conserved role of SREBP1 in rewiring cell metabolism in response to mechanical cues.
Genes & development 2009 OCT
Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A.
Yamaji D et al.
Abstract
Mammary alveologenesis is abrogated in the absence of the transcription factors STAT5A/5B, which mediate cytokine signaling. To reveal the underlying causes for this developmental block, we studied mammary stem and progenitor cells. While loss of STAT5A/5B did not affect the stem cell population and its ability to form mammary ducts, luminal progenitors were greatly reduced and unable to form alveoli during pregnancy. Temporally controlled expression of transgenic STAT5A in mammary epithelium lacking STAT5A/5B restored the luminal progenitor population and rescued alveologenesis in a reversible fashion in vivo. Thus, STAT5A is necessary and sufficient for the establishment of luminal progenitor cells.
PloS one 2009 JAN
MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors.
Teissedre B et al.
Abstract
Canonical Wnt/beta-catenin signaling regulates stem/progenitor cells and, when perturbed, induces many human cancers. A significant proportion of human breast cancer is associated with loss of secreted Wnt antagonists and mice expressing MMTV-Wnt1 and MMTV-DeltaN89beta-catenin develop mammary adenocarcinomas. Many studies have assumed these mouse models of breast cancer to be equivalent. Here we show that MMTV-Wnt1 and MMTV-DeltaN89beta-catenin transgenes induce tumors with different phenotypes. Using axin2/conductin reporter genes we show that MMTV-Wnt1 and MMTV-DeltaN89beta-catenin activate canonical Wnt signaling within distinct cell-types. DeltaN89beta-catenin activated signaling within a luminal subpopulation scattered along ducts that exhibited a K18(+)ER(-)PR(-)CD24(high)CD49f(low) profile and progenitor properties. In contrast, MMTV-Wnt1 induced canonical signaling in K14(+) basal cells with CD24/CD49f profiles characteristic of two distinct stem/progenitor cell-types. MMTV-Wnt1 produced additional profound effects on multiple cell-types that correlated with focal activation of the Hedgehog pathway. We document that large melanocytic nevi are a hitherto unreported hallmark of early hyperplastic Wnt1 glands. These nevi formed along the primary mammary ducts and were associated with Hedgehog pathway activity within a subset of melanocytes and surrounding stroma. Hh pathway activity also occurred within tumor-associated stromal and K14(+)/p63(+) subpopulations in a manner correlated with Wnt1 tumor onset. These data show MMTV-Wnt1 and MMTV-DeltaN89beta-catenin induce canonical signaling in distinct progenitors and that Hedgehog pathway activation is linked to melanocytic nevi and mammary tumor onset arising from excess Wnt1 ligand. They further suggest that Hedgehog pathway activation maybe a critical component and useful indicator of breast tumors arising from unopposed Wnt1 ligand.
Cell 2009 AUG
Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells.
Shimono Y et al.
Abstract
Human breast tumors contain a breast cancer stem cell (BCSC) population with properties reminiscent of normal stem cells. We found 37 microRNAs that were differentially expressed between human BCSCs and nontumorigenic cancer cells. Three clusters, miR-200c-141, miR-200b-200a-429, and miR-183-96-182 were downregulated in human BCSCs, normal human and murine mammary stem/progenitor cells, and embryonal carcinoma cells. Expression of BMI1, a known regulator of stem cell self-renewal, was modulated by miR-200c. miR-200c inhibited the clonal expansion of breast cancer cells and suppressed the growth of embryonal carcinoma cells in vitro. Most importantly, miR-200c strongly suppressed the ability of normal mammary stem cells to form mammary ducts and tumor formation driven by human BCSCs in vivo. The coordinated downregulation of three microRNA clusters and the similar functional regulation of clonal expansion by miR-200c provide a molecular link that connects BCSCs with normal stem cells.
Cancer research 2008 MAY
Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors.
Shafee N et al.
Abstract
The majority of BRCA1-associated breast cancers are basal cell-like, which is associated with a poor outcome. Using a spontaneous mouse mammary tumor model, we show that platinum compounds, which generate DNA breaks during the repair process, are more effective than doxorubicin in Brca1/p53-mutated tumors. At 0.5 mg/kg of daily cisplatin treatment, 80% primary tumors (n = 8) show complete pathologic response. At greater dosages, 100% show complete response (n = 19). However, after 2 to 3 months of complete remission following platinum treatment, tumors relapse and become refractory to successive rounds of treatment. Approximately 3.8% to 8.0% (mean, 5.9%) of tumor cells express the normal mammary stem cell markers, CD29(hi)24(med), and these cells are tumorigenic, whereas CD29(med)24(-/lo) and CD29(med)24(hi) cells have diminished tumorigenicity or are nontumorigenic, respectively. In partially platinum-responsive primary transplants, 6.6% to 11.0% (mean, 8.8%) tumor cells are CD29(hi)24(med); these populations significantly increase to 16.5% to 29.2% (mean, 22.8%; P textless 0.05) in platinum-refractory secondary tumor transplants. Further, refractory tumor cells have greater colony-forming ability than the primary transplant-derived cells in the presence of cisplatin. Expression of a normal stem cell marker, Nanog, is decreased in the CD29(hi)24(med) populations in the secondary transplants. Top2A expression is also down-regulated in secondary drug-resistant tumor populations and, in one case, was accompanied by genomic deletion of Top2A. These studies identify distinct cancer cell populations for therapeutic targeting in breast cancer and implicate clonal evolution and expansion of cancer stem-like cells as a potential cause of chemoresistance.
Nature 2006 JAN
Generation of a functional mammary gland from a single stem cell.
Shackleton M et al.
Abstract
The existence of mammary stem cells (MaSCs) has been postulated from evidence that the mammary gland can be regenerated by transplantation of epithelial fragments in mice. Interest in MaSCs has been further stimulated by their potential role in breast tumorigenesis. However, the identity and purification of MaSCs has proved elusive owing to the lack of defined markers. We isolated discrete populations of mouse mammary cells on the basis of cell-surface markers and identified a subpopulation (Lin-CD29hiCD24+) that is highly enriched for MaSCs by transplantation. Here we show that a single cell, marked with a LacZ transgene, can reconstitute a complete mammary gland in vivo. The transplanted cell contributed to both the luminal and myoepithelial lineages and generated functional lobuloalveolar units during pregnancy. The self-renewing capacity of these cells was demonstrated by serial transplantation of clonal outgrowths. In support of a potential role for MaSCs in breast cancer, the stem-cell-enriched subpopulation was expanded in premalignant mammary tissue from MMTV-wnt-1 mice and contained a higher number of MaSCs. Our data establish that single cells within the Lin-CD29hiCD24+ population are multipotent and self-renewing, properties that define them as MaSCs.
View All Publications

 网站首页
网站首页