概要
Using appropriate cytokines (e.g. StemSpan™ CC100, StemSpan™ CC110, or StemSpan™ CD34+ Expansion Supplement), StemSpan™ SFEM II can be used for the expansion of total nucleated cells and CD34+ cells from cord blood, bone marrow, or other cell sources. StemSpan™ SFEM II can also be used to expand and differentiate lineage-committed progenitor cells to generate erythroblasts, granulocytes, monocytes, or megakaryocytes when used with StemSpan™ Erythroid Expansion Supplement (Catalog #02692), StemSpan™ Myeloid Expansion Supplement (Catalog #02693), StemSpan™ Myeloid Expansion Supplement II (Catalog #02694), or StemSpan™ Megakaryocyte Expansion Supplement (Catalog #02696), respectively.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™ SFEM II | 09605, 09655 | All | English |
| Safety Data Sheet | StemSpan™ SFEM II | 09605, 09655 | All | English |
数据及文献
Data
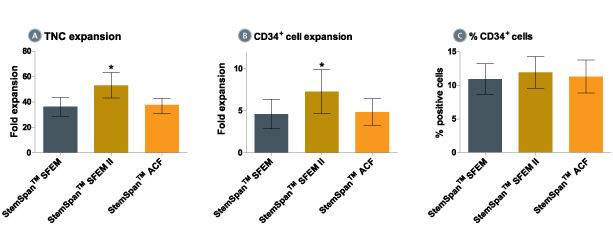
Figure 1. Expansion of CD34 + Human Cord Blood Cells Cultured in StemSpan™ Media Containing CC100 Cytokine Cocktail
Purified CD34 + human cord blood (CB) cells were suspended at a concentration of 10,000 per mL in StemSpan™ SFEM (dark gray bars), SFEM II (gold bars) and ACF (orange bars) media containing CC100 Cytokine Cocktail (Catalog #02690). Cultures were maintained for 7 days, after which the cells were counted and examined for CD34 and CD45 expression by flow cytometry. Shown are the fold expansion of total nucleated cells (TNC) (A) and CD34 + cells (B) per input CD34 + cell, and the percent CD34 + cells (C). Results represent the average results of 32 different CB samples. Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in StemSpan™ SFEM and StemSpan™-ACF (*p<0.001, paired t-test, n=32).
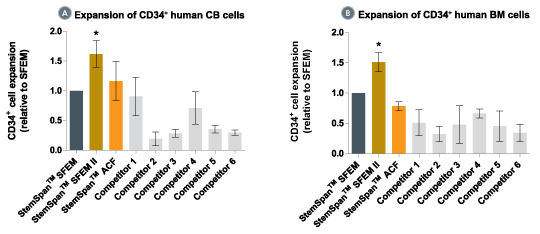
Figure 2. StemSpan™ SFEM II Serum-Free Expansion Medium Containing CC100 Cytokine Cocktail Supports Greater Expansion of Human CD34 + Cells Than Other Media Tested
Expansion of CD34 + cells, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bars) after culturing purified CD34 + CB (A, n=6) or bone marrow (BM) (B, n=3) cells for 7 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, StemPro34 (Life Technologies), X-Vivo-15 and HPGM (both from Lonza), SCGM (Cellgenix), StemLine II (Sigma) and HP01 (Macopharma)). All media were supplemented with StemSpan™ CC100 Cytokine Cocktail (Catalog #02690). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of CB and BM cells produced in StemSpan™ SFEM II were significantly higher than in all other media, except the numbers of CB cells produced in StemSpan™-ACF (*p<0.05, paired t-test).
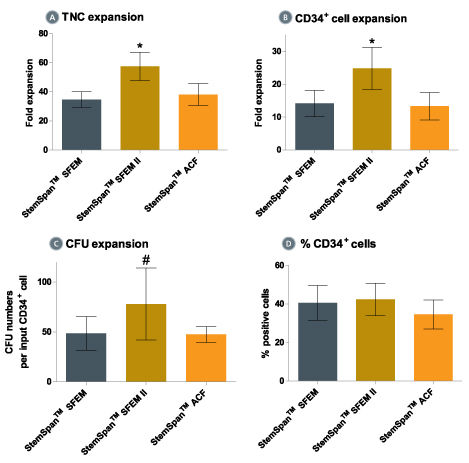
Figure 3. Expansion of CD34 + Human Cord Blood Cells Cultured in StemSpan™ Media Containing CD34 + Expansion Supplement
Purified CD34 + human cord blood (CB) cells were suspended at a concentration of 10,000 per mL in StemSpan™ SFEM (dark gray bars), SFEM II (gold bars) and ACF (orange bars) media containing CD34 + Expansion Supplement (Catalog #02691). Cultures were maintained for 7 days, after which the cells were counted and examined for CD34 and CD45 expression by flow cytometry. The number of colony-forming units (CFU) in the expanded population was determined by replating cells in MethoCult™ H4435 and counting the number of colonies produced 14 days later. Shown are the fold expansion of total nucleated cells (TNC) (A), CD34 + cells (B) and CFU numbers (C) per input CD34 + cell, and the percent CD34 + cells (D) in these cultures (n=6). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II was significantly higher than in SFEM and ACF (*p<0.001, #p<0.05, paired t-test, n=6).
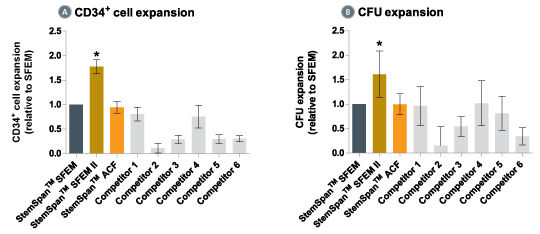
Figure 4. StemSpan™ SFEM II Serum-Free Expansion Medium Containing CD34 + Expansion Supplement Supports Greater Expansion of Human CD34 + Cells Than Other Media Tested
Expansion of CD34 + cells (A) and CFUs (B), normalized relative to the values obtained in SFEM medium (dark gray bars) after culturing purified CD34 + CB cells for 7 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other suppliers (light gray bars, Competitor 1-6, which included, in random order, X-Vivo-15 (Lonza), HP01 (Macopharma), StemPro34 (Life Technologies), SCGM (Cellgenix), StemLine II (Sigma), and HPGM (Lonza). All media were supplemented with the StemSpan™ CD34 + Expansion Supplement (Catalog #02691). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in all other media (*p<0.01, paired t-test, n=6).
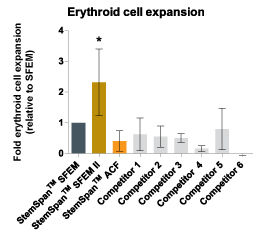
Figure 5. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Erythroid Expansion Supplement Supports Greater Expansion of Erythroid Cells Than Other Media Tested
The numbers of erythroid cells, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar), obtained after culturing purified CD34 + CB cells for 14 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, X-Vivo-15 and HPGM (both from Lonza), StemLine II (Sigma), HP01 (Macopharma), StemPro34 (Life Technologies) and SCGM (Cellgenix). All media were supplemented with StemSpan™ Erythroid Expansion Supplement (Catalog #02692). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in all other media (*p<0.05, paired t-test, n=6).
Table 1. Production of Myeloid Cells from Human CB CD34+ Cells Cultured in SFEM II Containing Myeloid Expansion Supplement or Myeloid Expansion Supplement ll
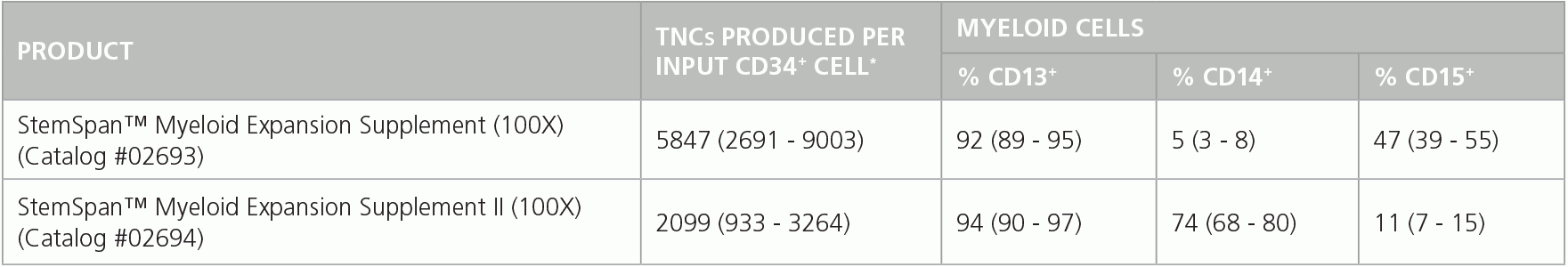
Shown are numbers of total nucleated cells (TNCs) produced per input human CB-derived CD34+ cell and percentages of cells positive for myeloid markers CD13, CD14 and CD15 after 14 days of culture in SFEM II containing Myeloid Expansion Supplement (n = 14) or Myeloid Expansion Supplement II (n = 16). *95% confidence limits (CL); the range within which 95% of results typically fall.
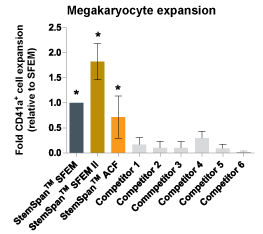
Figure 6. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Megakaryocyte Expansion Supplement Supports Greater Expansion of Megakaryocytes Than Other Media Tested
The numbers of megakaryocytes, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar), obtained after culturing purified CD34 + CB cells for 14 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, StemLine II (Sigma), HPGM (Lonza), HP01 (Macopharma), SCGM (Cellgenix), StemPro34 (Life Technologies) and X-Vivo-15 (Lonza). All media were supplemented with StemSpan™ Megakaryocyte Expansion Supplement (Catalog #02696). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in the StemSpan™ media were significantly higher than in the other media (*p<0.01 paired t-test, n=6).

Figure 7. StemSpan™ SFEM II Serum-Free Expansion Medium Containing T Cell Progenitor Expansion Supplement Promotes the Expansion and Differentiation of CB-Derived CD34+ Cells into Pro- and Pre-T Cells
The average (A,C) frequencies and (B,D) numbers of (A,B) CD7+CD5+ pro-T cells and (C,D) CD7+CD1a+ pre-T cells on days 7, 14 and 21 of culture with the StemSpan™ T Cell Progenitor Differentiation Kit (Catalog #09900) are shown for 10 - 26 independent experiments. The average frequency of (A) pro- and (C) pre-T cells were 84% and 28% respectively, after 21 days of culture. All pro- and pre-T cells were found to express intracellular CD3 (data not shown). The number of (B) CD7+CD5+ pro-T cells increased (on average) ~10 - 100-fold every week, resulting in an average number of ~2100 pro-T cells produced per input CD34+ cell on day 21. After 21 days of culture (D) pre-T cells expressing CD7 and CD1a are present in large numbers, indicating the further differentiation of pro-T cells. The yield of (D) CD7+CD1a+ cells on day 21 was ~800 per input CD34+ cell. BM-derived CD34+ cells also expanded and differentiated to pro-T cells in stroma-free cultures with approximately 70 CD7+CD5+ cells produced per input CD34+ cell at day 21 (n = 3; data not shown). Vertical lines indicate 95% confidence limits (CL), the range within which 95% of results typically fall.

 网站首页
网站首页