概要
技术资料
Scientific Resources
Product Documentation
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™ Leukemic Cell Culture Kit | 09720 | All | English |
| Product Information Sheet | StemSpan™ SFEM II | 09605 | All | English |
| Product Information Sheet | StemSpan™ CD34+ Expansion Supplement (10X) | 02691 | All | English |
| Product Information Sheet | UM729 | 72332 | All | English |
| Safety Data Sheet 1 | StemSpan™ Leukemic Cell Culture Kit | 09720 | 全部 | English |
| Safety Data Sheet 2 | StemSpan™ Leukemic Cell Culture Kit | 09720 | 全部 | English |
| Safety Data Sheet | StemSpan™ SFEM II | 09605 | 全部 | English |
| Safety Data Sheet | UM729 | 72332 | 全部 | English |
数据及文献
Data
The experiments in this Technical Bulletin were performed using cryopreserved cells, however similar results are expected when using fresh samples. Additionally in place of UM729, the small molecule UM171 was used to generate data in Figures 4 - 7. UM171 is no longer licensed for sale by STEMCELL Technologies, however similar results are expected when using UM729 prepared to a final concentration of 1 μM (data not shown). Further titration may be necessary to optimize cell fold expansion in specific conditions.For more information including data comparing UM171 and UM729, see Fares et al. 2014.
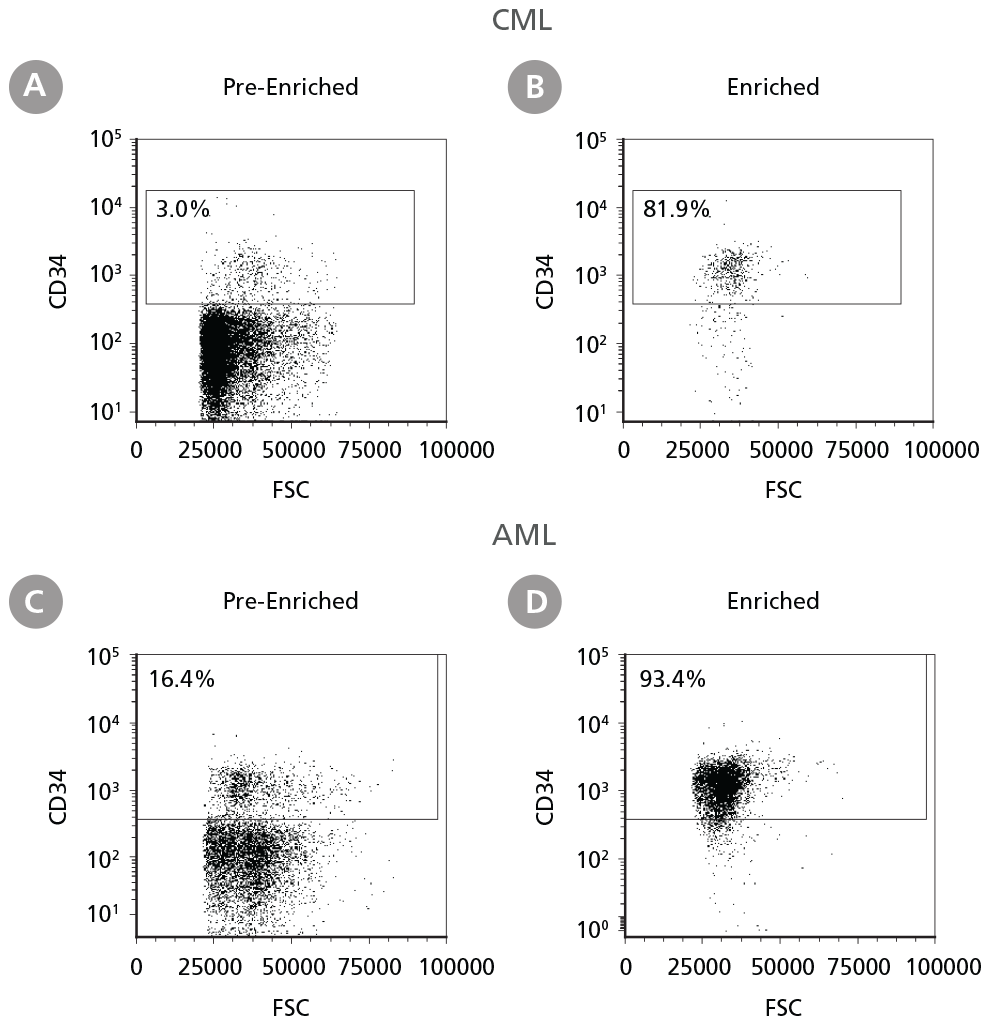
Figure 1. Isolation of Leukemic CD34+ Cells
Cryopreserved CML or AML PBMCs and BMMCs were thawed and prepared for isolation. CD34+ cells were isolated using EasySep™ Human Cord Blood CD34 Positive Selection Kit II. The percentage of CD34+ cells before (A, C) and after (B, D) CD34+ cell isolation was measured by flow cytometry. Dead cells were excluded by light scatter profile and viability staining. In this example the purity of CD34+ cells increased from 3% to 82% (CML) and from 16% to 93% (AML).
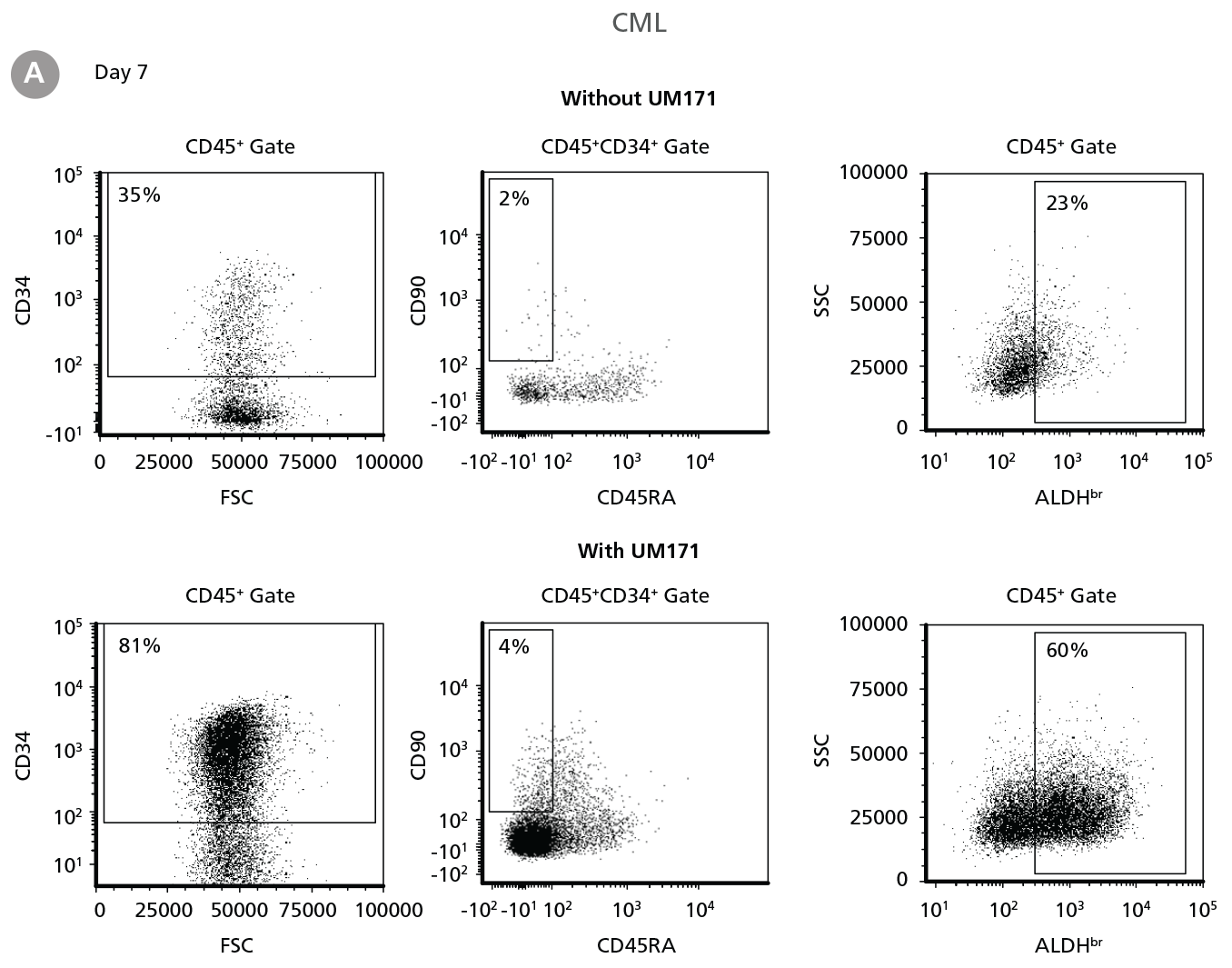

Figure 2. Expansion of CD34+ CML Cells
CD34+ CML cells were cultured in StemSpan™ SFEM II containing CD34+ Expansion Supplement (Exp) without or with UM171. After 7 and 14 days, the cultured cells were stained with fluorescently labeled antibodies against CD45, CD34, CD90, CD45RA, and with ALDEFLUOR™ (Catalog #01700) to measure ALDH activity, and analyzed by flow cytometry. Sequential gates were used to determine the percentages of viable CD45+, CD45+CD34+ and CD45+CD34+CD90+CD45RA- cells(based on “Fluorescence Minus One” (FMO) controls), and ALDHbr cells (based on DEAB control). (A) Representative flow cytometry profiles at day 7 are shown. The (B,D) frequency and (C,E) cell numbers of these subsets per initial CD34+ cell on (B,C) day 7 and (D,E) day 14 are shown. StemSpan™ SFEM II supplemented with CD34+ Expansion Supplement supports the expansion of CML cells in culture. The addition of UM171 enhances expansion of all subsets shown (~10-fold expansion of CD34+ progenitor cells at day 7 and ~20-fold at day 14 compared to cultures without UM171). Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.0001). All six CML samples tested were able to expand in culture.
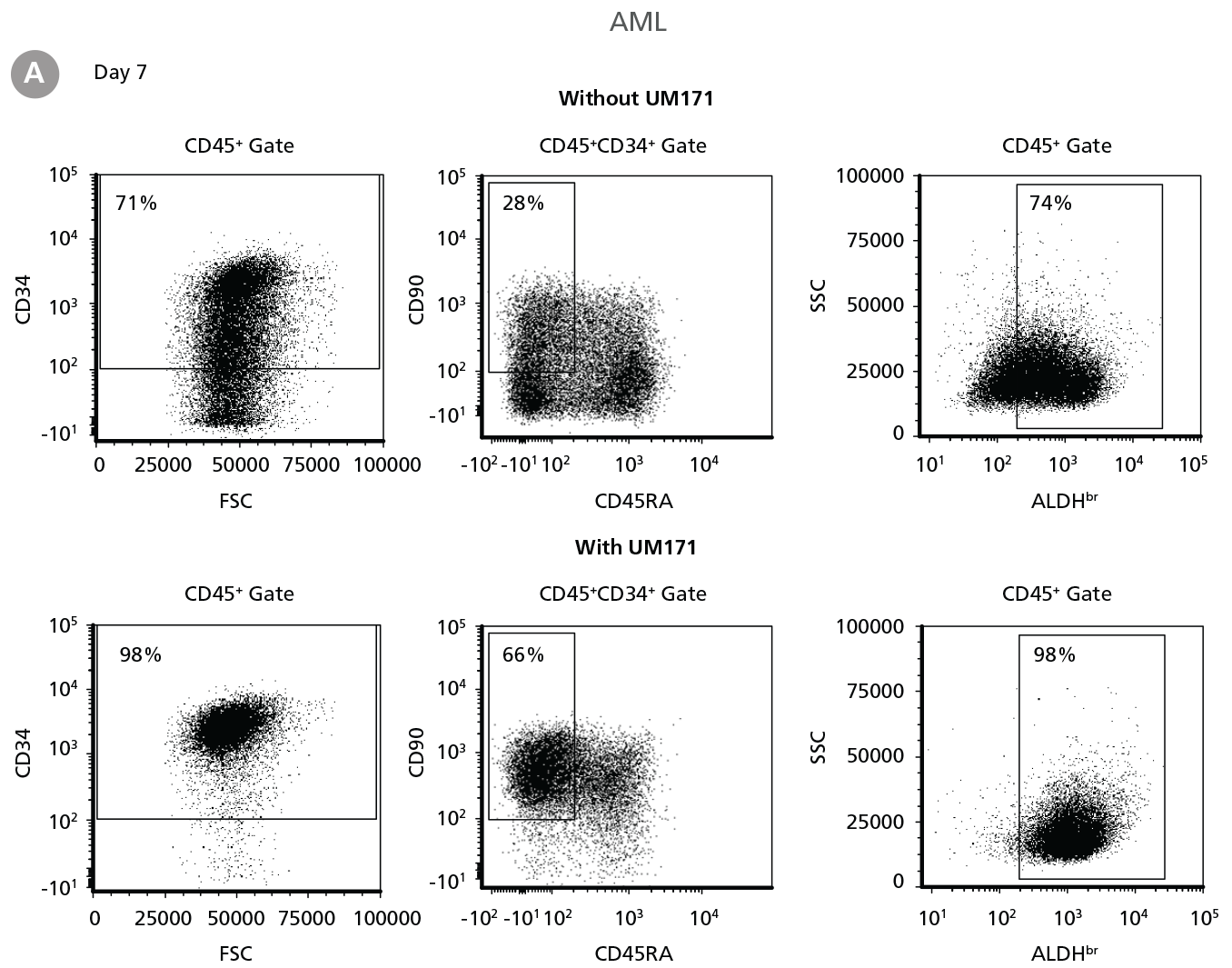
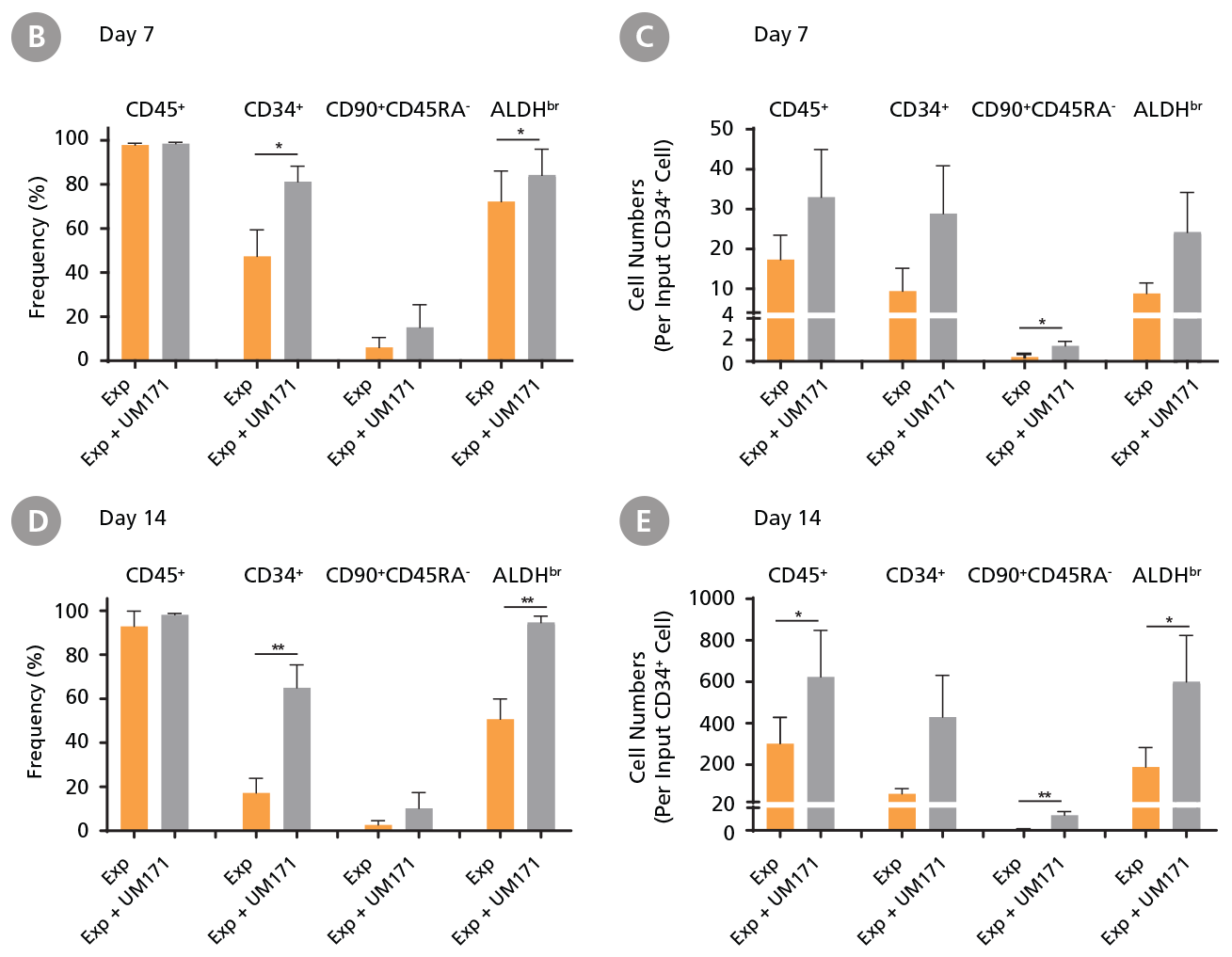
Figure 3. Expansion of CD34+ AML Cells
CD34+ AML cells were cultured in StemSpan™ SFEM II containing CD34+ Expansion Supplement (Exp) alone, or with UM171. After 7 and 14 days, the cultured cells were stained with fluorescently labeled antibodies and with ALDEFLUOR™ Reagent as described in Figure 2. (A) Representative flow cytometry profiles at day 7 are shown. The (B, D) frequency and (C, E) cell numbers of these subsets per initial CD34+ cell on (B, C) day 7 and (D, E) day 14 are shown. SFEM II supplemented with CD34+ Expansion Supplement supports the expansion of AML cells in culture. The addition of UM171 further enhances expansion of all subsets shown (~3-fold expansion of all subsets at day 7 and ~7-fold at day 14 compared to cultures without UM171). Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05; **P < 0.01). Six out of ten AML samples tested were able to expand in culture.

Figure 4. Colony-Forming Potential of CD34+ CML Cells is Maintained During Culture
CML cells were assayed in colony assays using MethoCult™ H4435 Enriched medium directly after CD34+ cell isolation (day 0) or after 7 or 14 days of expansion without or with UM171 (as described in Figure 2). After 14 days of culture in StemSpan™ SFEM II with CD34+ expansion supplement (Exp) with or without UM171, colonies were (A) imaged with STEMvision™ and counted manually from digital images. (B) CFU output expressed as the total number of colonies per original input CD34+ cell. Numbers above each of the individual bars indicate the proportion of BCR-ABL positive colonies, measured by qRT-PCR on individual plucked colonies across 6 different samples (8-12 colonies were plucked for each sample per condition). SFEM II supplemented with CD34+ Expansion Supplement (Exp) supports the expansion of colony-forming progenitor cells in culture. UM171 further promotes colony forming progenitor cell output (~3.5-fold expansion at day 7 and ~8-fold at day 14). Single-colony qRT-PCR analysis revealed that colonies generated from samples on day 0, and colonies generated from cells expanded for 7 and 14 days, were predominantly BCR-ABL+ but also that normal BCR-ABL- progenitor cells were present at low frequencies. Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05).

Figure 5. Colony-Forming Potential of CD34+ AML Cells is Maintained During Culture
AML cells, after CD34+ cell isolation (day 0) or after 7 or 14 days of expansion without or with UM171 (as described in Figure 3), were plated in colony assays with MethoCult™ H4435 Enriched medium. After 14 days of incubation, colonies were (A) imaged with STEMvision™ and counted manually from digital images. (B) CFU output expressed as the total number of colonies per original input CD34+ cell. SFEM II supplemented with CD34+ Expansion Supplement (Exp) supports the expansion of colony-forming progenitor cells in culture. Addition of UM171 further promotes colony-forming progenitor cell output (~3-fold expansion at day 7 and ~4-fold at day 14). Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05).

 网站首页
网站首页