概要
When added to serum-free medium, StemSpan™ Erythroid Expansion Supplement typically promotes the production of thousands of erythroid cells per input CD34+ cell in 14-day liquid cultures initiated with CD34+ human CB cells. See data tab for more details.
StemSpan™ Erythroid Expansion Supplement (100X) is intended for use in combination with any of the following StemSpan™ media:
• StemSpan™ SFEM (Catalog #09600)
• StemSpan™ SFEM II (Catalog #09605)
• StemSpan™-XF (Catalog #100-0073)
• StemSpan™-ACF Erythroid Expansion Medium (Catalog #09860)
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™ Erythroid Expansion Supplement (100X) | 02692 | All | English |
| Safety Data Sheet | StemSpan™ Erythroid Expansion Supplement (100X) | 02692 | All | English |
数据及文献
Data
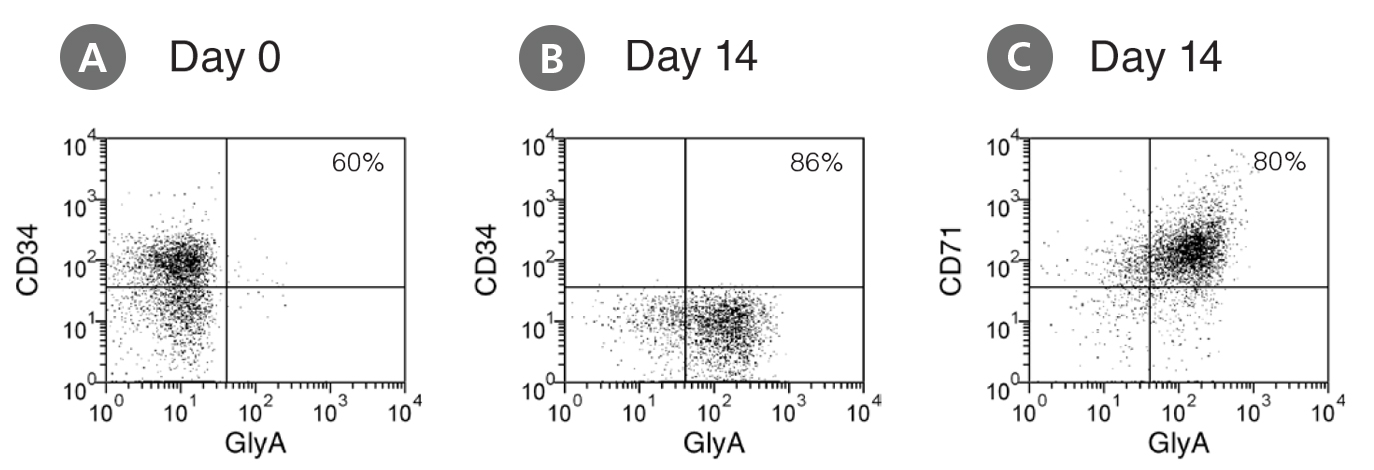
Figure 1. Production of Erythroblasts by Expansion and Lineage Specific Differentiation of CD34+ Human Cord Blood Cells Cultured in StemSpan™ SFEM Containing Erythroid Expansion Supplement
Flow cytometry dot plots showing expression of the HSPC marker CD34 and erythroid markers CD71 and glycophorin-A (GlyA) (A) before culture and (B,C) after 14 days of culture of enriched CD34+ CB cells in StemSpan™ SFEM containing Erythroid Expansion Supplement. The frequency of CD34+ cells declined from ~60% before culture to <0.1% after 14 days. In parallel, erythroid cells gradually accumulated from levels of <1% before culture to >90% by day 14. The bulk of cell population recovered from 14-day culture consisted of CD71+GlyA+ erythroblasts. More immature CD71+GlyA- progenitors and proerythroblasts, as well as more differentiated CD71-/low GlyA+ normoblasts were also present at low frequencies.
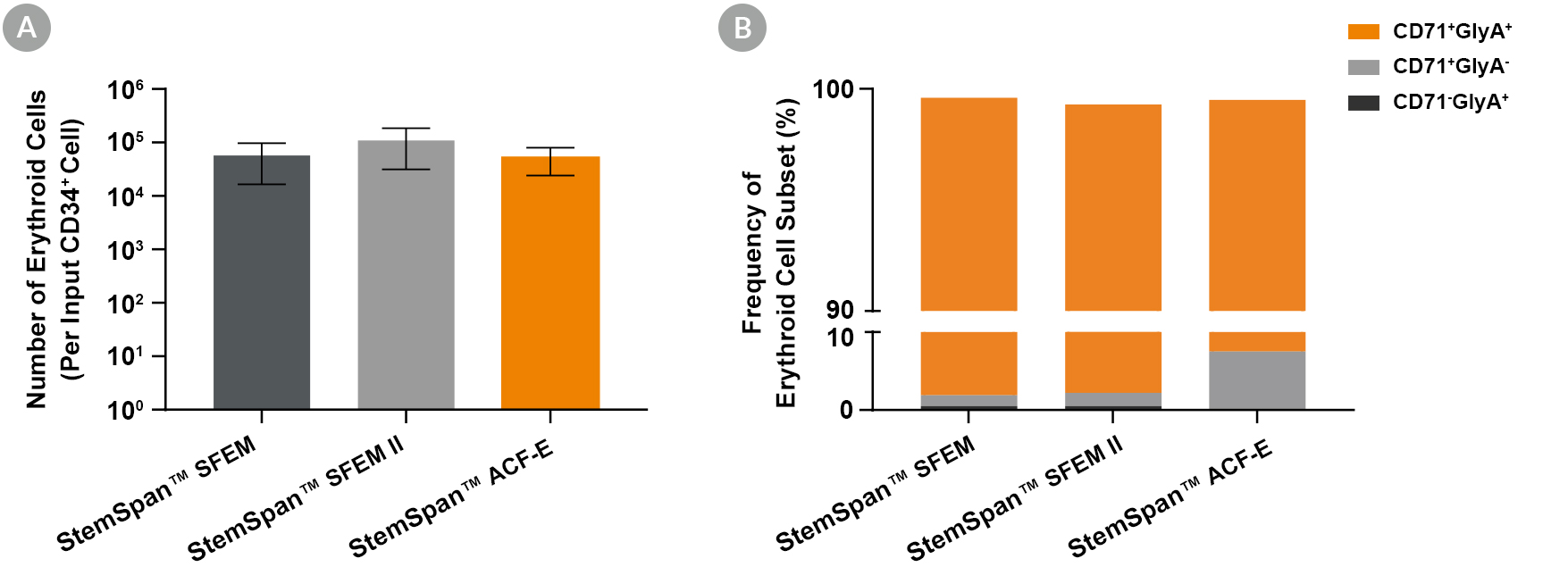
Figure 2. Production of Erythroid Cells from Human CB-Derived CD34+ Cells Cultured in StemSpan™ Media Containing StemSpan™ Erythroid Expansion Supplement
(A) Average numbers of erythroid cells generated after culturing purified CD34+ CB cells (n = 5) for 14 days in StemSpan™ SFEM (black bars), SFEM II (grey bars), or StemSpan™-ACF Erythroid Expansion Medium (ACF-E, orange bars) media containing StemSpan™ Erythroid Expansion Supplement (Catalog #02692). Shown are the number of erythroid cells that express CD71 and/or Glycophorin A (GlyA) per input CD34+ cell. (B) The percentages of the different erythroid cell subsets generated in these cultures are shown, including CD71+GlyA+ erythroblasts, immature CD71+GlyA- erythroid progenitor cells and pro-erythroblasts, and CD71-/lowGlyA+ normoblasts. All three media supported the generation of thousands of erythroid cells per CB-derived CD34+ cell plated.
Table 1. Production of Erythroid Cells from Human CB-Derived CD34+ Cells Cultured in StemSpan™ Media Containing StemSpan™ Erythroid Expansion Supplement
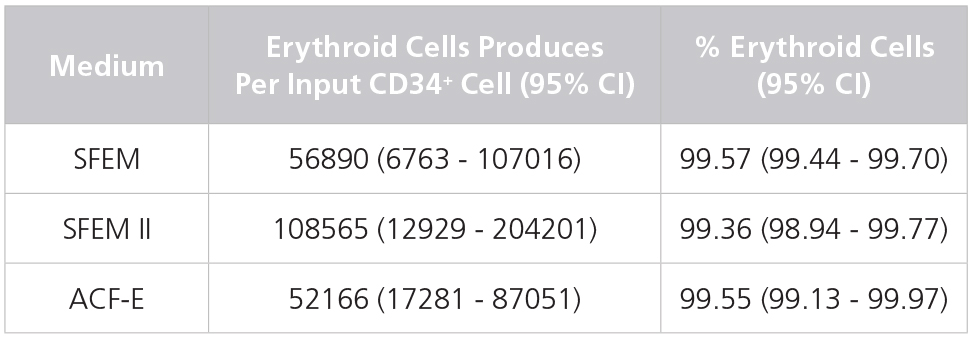
Yields and percentages of erythroid cells produced after culturing purified CD34+ CB cells (n = 5) for 14 days in StemSpan™ SFEM, SFEM II or ACF-E media containing Erythroid Expansion Supplement. Erythroid cells were identified by flow cytometry after staining with antibodies against CD71 and GlyA. The % erythroid cells represent the percentage of cells that express CD71 and/or GlyA. *CI: Confidence Interval.

 网站首页
网站首页