概要
Using appropriate StemSpan™ Expansion Supplements, StemSpan™-XF may be used to expand CD34+ cells isolated from human cord blood, mobilized peripheral blood, or bone marrow samples, or to expand and differentiate lineage-committed progenitor cells to generate populations of erythroid, myeloid, or megakaryocyte progenitor cells.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™-XF | 100-0073 | All | English |
| Safety Data Sheet | StemSpan™-XF | 100-0073 | All | English |
数据及文献
Data
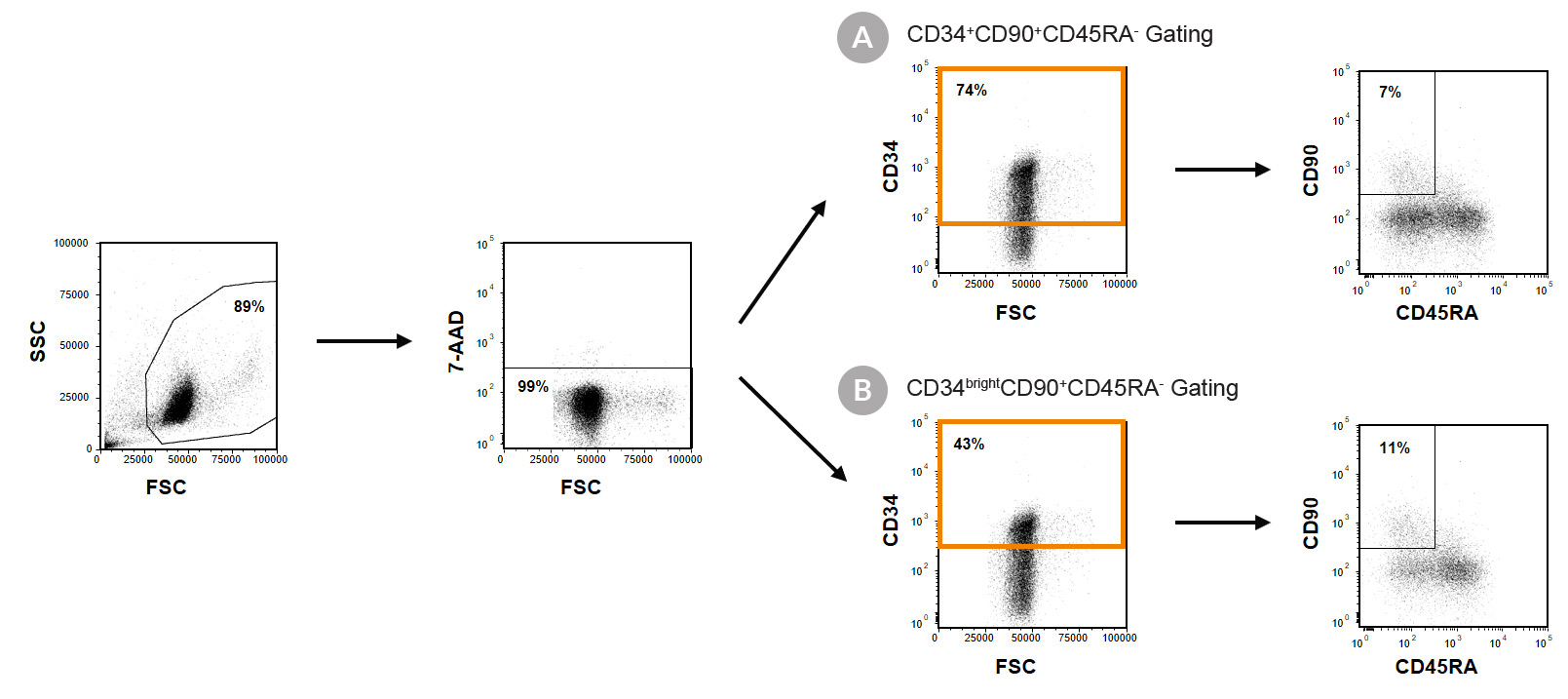
Figure 1. Immunophenotyping of Cultured CD34+ Cells
Cord blood (CB)-derived CD34+ cells were purified using EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896) and cultured in StemSpan™-XF (Catalog #100-0073) supplemented with StemSpan™ CD34+ Expansion Supplement (Catalog #02691) and UM171*. After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD90 and CD45RA, in addition to viability dye 7-AAD, and analyzed by flow cytometry. Sequential gates were used to determine the percentages of viable (A) CD34+ and CD34+CD90+CD45RA- cells, based on fluorescence minus one (FMO) controls and (B) CD34bright and CD34brightCD90+CD45RA- cells.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
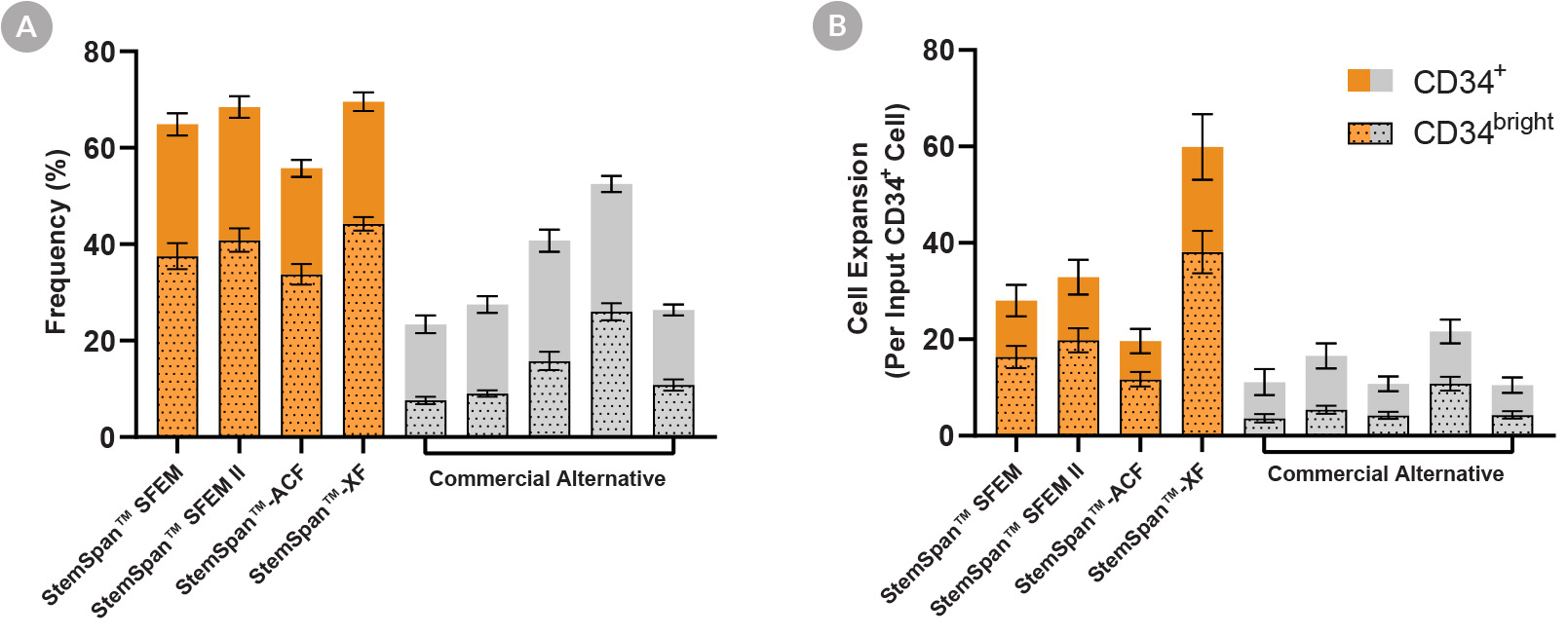
Figure 2. StemSpan™ Media Support Greater Expansion of Human CD34+ and CD34bright Cells Than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in StemSpan™ media (SFEM, SFEM II, ACF, and XF, orange bars), and in five media from other suppliers (Commercial Alternative, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of viable CD34+ and CD34bright cells in culture were analyzed by flow cytometry as described in Figure 1. Compared to the commercial alternatives tested, StemSpan™ media showed significantly higher expansion of CD34+ and CD34bright cells (P < 0.05 when comparing StemSpan™ SFEM, SFEM II, and XF to five media from other suppliers, calculated using a one-way ANOVA followed by Dunnett’s post hoc test). The CD34bright cell population is enriched for functional stem/progenitor cells (See Figure 4). Data shown are mean ± SEM (n = 8).
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
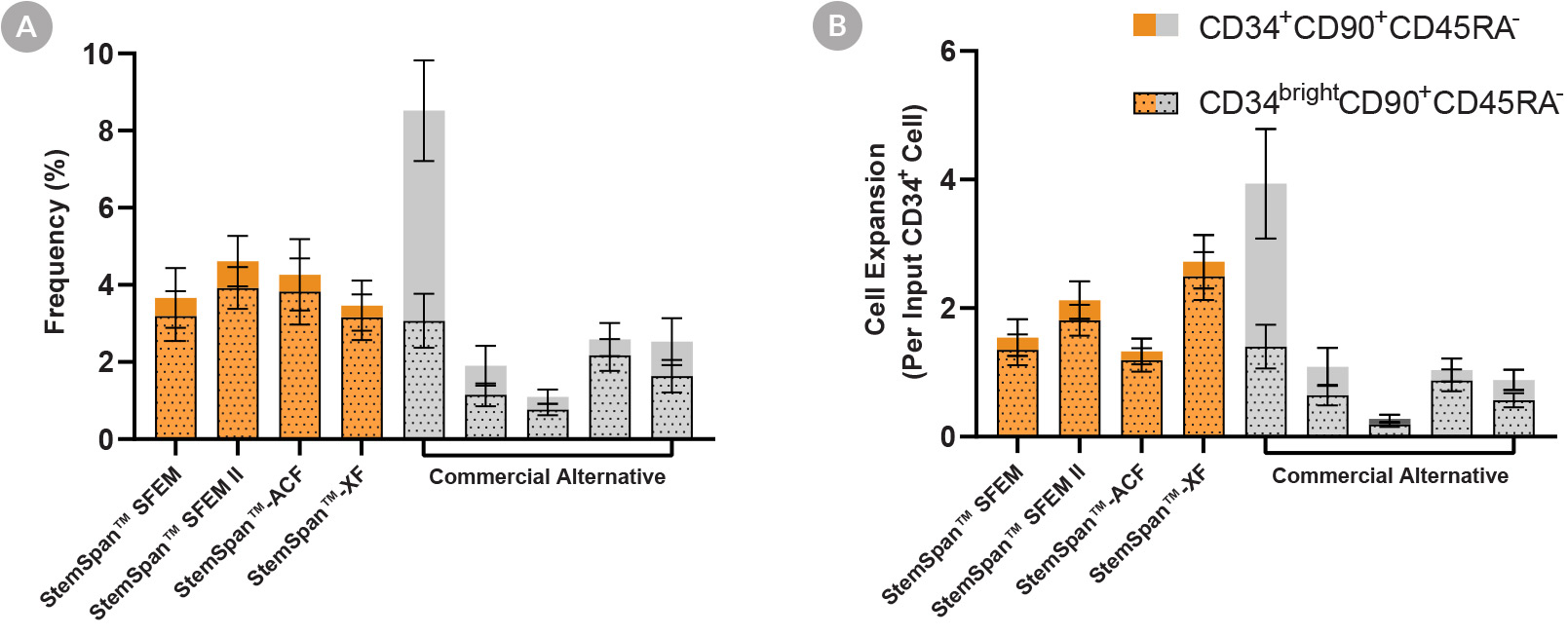
Figure 3. StemSpan™ Media Support Equal or Greater Expansion of Primitive Human CD34brightCD90+CD45RA- Cells Than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in StemSpan™ media (SFEM, SFEM II, ACF, and XF, orange bars), and in five media from other suppliers (Commercial Alternative, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of viable CD34+CD90+CD45RA- (solid) and CD34brightCD90+CD45RA- (dotted overlay) cells in culture were analyzed by flow cytometry as described in Figure 1. Compared to the competitor media tested, StemSpan™ media showed similar or significantly higher expansion of CD34brightCD90+CD45RA- cells (P < 0.05 when comparing StemSpan™ SFEM II and XF to five media from other suppliers, calculated using one-way ANOVA followed by Dunnett’s post hoc test). The CD34brightCD90+CD45RA- cell population is highly enriched for functional stem/progenitor cells (See Figure 4). Data shown are mean ± SEM (n = 8).
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
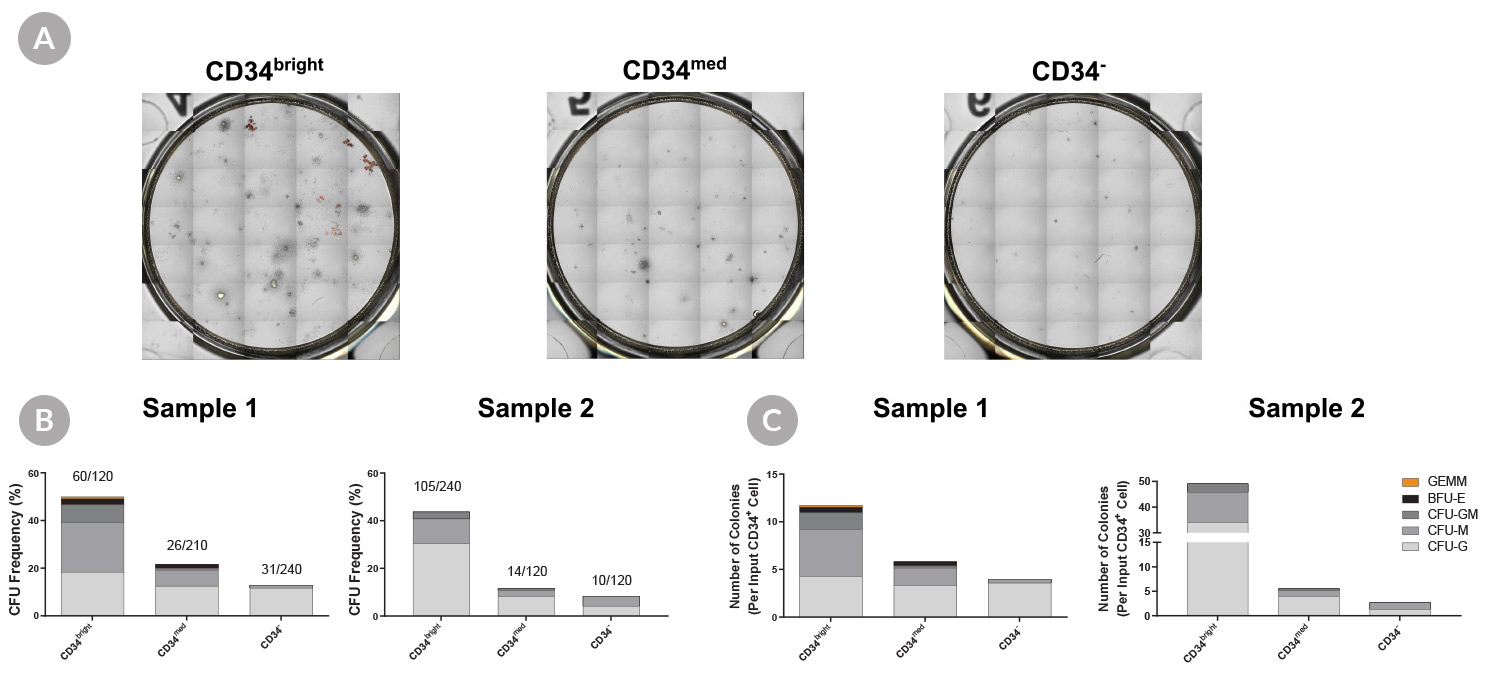
Figure 4. Culture-Expanded CD34bright Cells Have Higher Colony-Forming Potential Than Culture-Expanded CD34med and CD34- Cells
Purified CB-derived CD34+ cells (n = 2) were cultured in StemSpan™-XF with StemSpan™ CD34+ Expansion Supplement and UM171* for 7 days. Cultured cells were stained with an anti-CD34 antibody and 7-AAD for sorting. Anti-CD90, and CD45RA antibodies were also included, to be used in later analyses. Cells were either (A) bulk-sorted or (B,C) single cell index-sorted from CD34bright, CD34med, and CD34- populations. Sorted cells were plated in colony-forming unit (CFU) assays using MethoCult™ H4034 Optimum (Catalog #04034). (A) Bulk-sorted cells were plated at 400 cells/mL in 6-well SmartDish™ plates (Catalog #27370) and (B,C) single cells were sorted directly into 96-well plates (one cell per well, prefilled with 100 µL of MethoCult™). After 14 days of incubation, colonies were counted with (A) STEMvision™ or (B,C) manually using an inverted microscope. (A) Shown are representative images of CFU assays from bulk-sorted cells of CD34bright, CD34med, and CD34- populations. The frequency of colonies in each population from the single cell-sorted experiments are shown as (B) CFU frequency, calculated as the total number of colonies generated by the total number of individually sorted cells (shown respectively as a fraction above each bar), and (C) CFU output expressed as total numbers of CFU in each sorted population per original input CD34+ cell.
The culture-expanded CD34bright cells showed (B) higher CFU frequency (~50%) than CD34med cells (~20%) and CD34- cells (~10%), and yielded more diverse and primitive colony types, including CFU-GEMM and BFU-E colonies, which were rarely generated from CD34med and CD34- cells. When phenotyping data, collected during sorting, was analyzed all CFU-GEMM and the majority of of BFU-E colonies were generated from the fraction enriched for LT-HSCs, the CD34brightCD90+CD45RA- cell population, while 33% of BFU-E colonies were generated from the fraction enriched for ST-HSCs, the CD34brightCD90-CD45RA- cell population. Only small CFU-G and CFU-M colonies were generated from CD34brightCD90+/-CD45RA+ cells, which is consistent with publications suggesting this population contains only progenitor cells.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.

 网站首页
网站首页