概要
StemSpan™-AOF contains only recombinant proteins and synthetic components, and does not contain serum or other human- or animal-derived components.
Using appropriate cytokines, StemSpan™-AOF may be used to expand CD34+ cells isolated from human cord blood, mobilized peripheral blood, or bone marrow samples, or to expand and differentiate lineage-committed progenitor cells to generate populations of myeloid or megakaryocyte progenitor cells.
Please note, StemSpan™-AOF was originally launched as StemSpan™-ACF Without Phenol Red. This name change signifies that in addition to being animal component-free, no materials of animal or human origin are used in the manufacture of this medium or its components, to at least the secondary level of manufacturing. This medium also replaces StemSpan™-ACF (Catalog #09855).
StemSpan™-AOF (Catalog #100-0130) is manufactured under relevant cGMPs, ensuring the highest quality and consistency for reproducible results. For additional quality information, visit www.STEMCELL.com/compliance.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™-AOF | 100-0130 | All | English |
| Safety Data Sheet | StemSpan™-AOF | 100-0130 | All | English |
数据及文献
Data
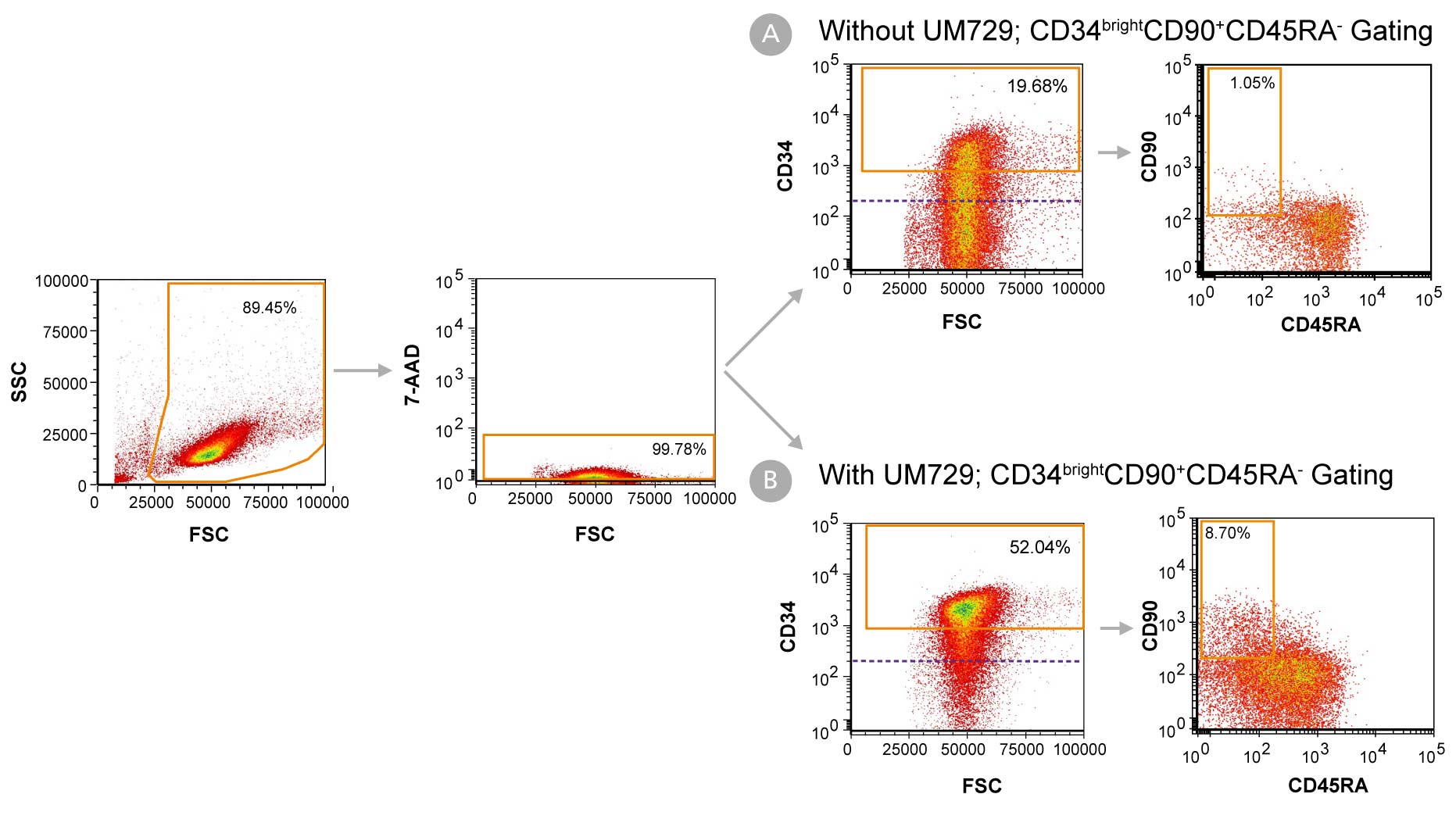
Figure 1. Day 7 Immunophenotyping of CD34+ Cells Cultured in StemSpan™-AOF
CD34+ cells were purified from cord blood (CB) using the EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896) and cultured in StemSpan™-AOF (Catalog #100-0130) supplemented with StemSpan™ CD34+ Expansion Supplement (Catalog #02691) (A) without or (B) with the addition of UM729 (Catalog #72332). After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD90, and CD45RA, in addition to viability dye 7-AAD, and analyzed by flow cytometry. The horizontal dotted line in the CD34 vs FSC plots indicates the boundary between CD34- and CD34+ cells as based on a fluorochrome minus one (FMO) control for CD34 expression. Orange gates on these plots indicate the population of CD34bright cells used to generate data in Figures 2 and 3. Sequential gates were used to determine the percentages of viable CD34+ cells, CD34bright cells, and CD34brightCD90+CD45RA- cells.
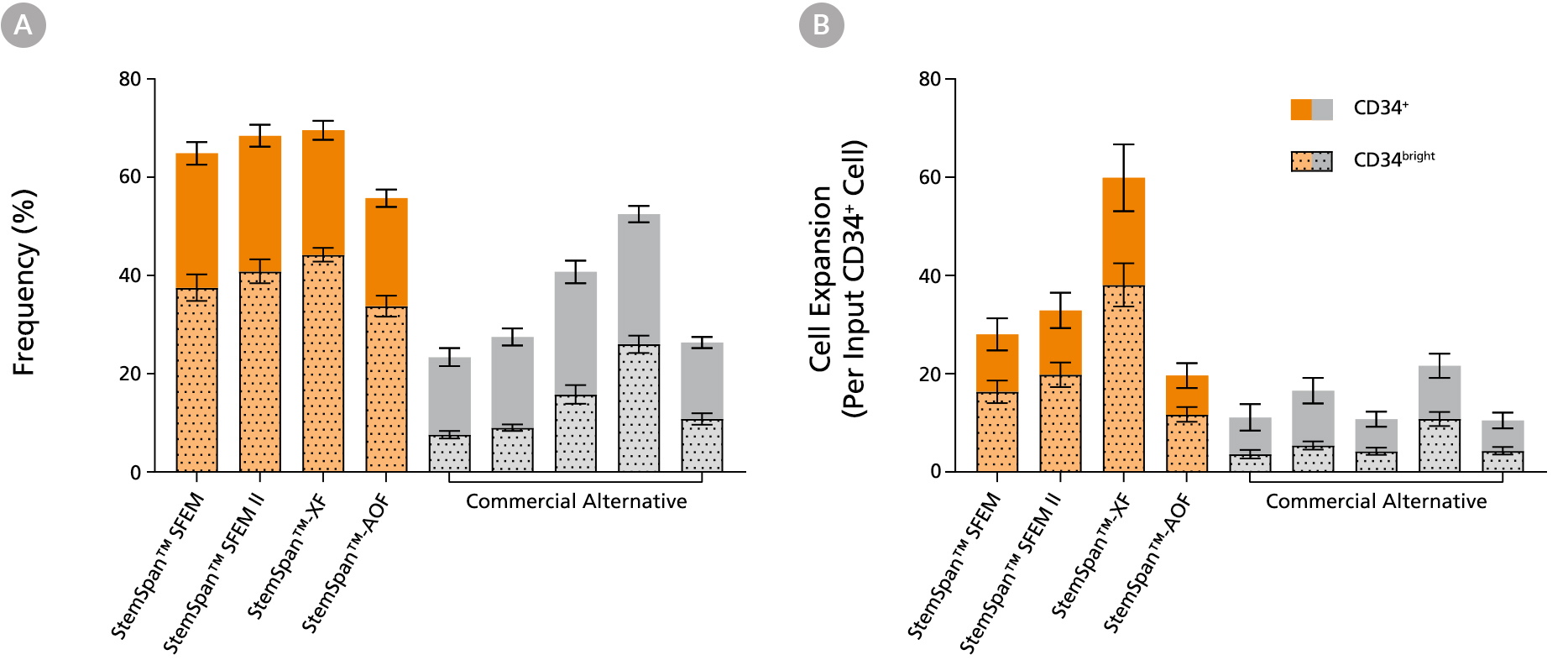
Figure 2. StemSpan™ Media Support Greater Expansion of Human CD34+ and CD34bright Cells than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in select StemSpan™ media (StemSpan™ SFEM, StemSpan™ SFEM II, StemSpan™-XF, or StemSpan™-AOF, orange bars), and in five xeno-free media formulations from other suppliers (Xeno-Free Commercial Alternative, grey bars) including (in random order) CTS™ StemPro™ HSC (Thermo), SCGM (Cellgenix), X-VIVO™ 15 (Lonza), Stemline™ II (Sigma), and StemPro™-34 (Thermo). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of viable CD34+ and CD34bright cells in culture were based on viable cell counts and flow cytometry results as shown in Figure 1. StemSpan™ showed significantly higher expansion of CD34+ and CD34bright cells (P < 0.05 when comparing StemSpan™ SFEM II to five media from other suppliers, calculated using a one-way ANOVA followed by Dunnett’s post hoc test) and StemSpan™-AOF, the only animal origin-free formulation, showed equivalent performance to all xeno-free competitors tested. Data shown are mean ± SEM (n = 8).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version StemSpan™-ACF (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version, StemSpan™-AOF (Catalog #100-0130) was comparable.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al., 2014.
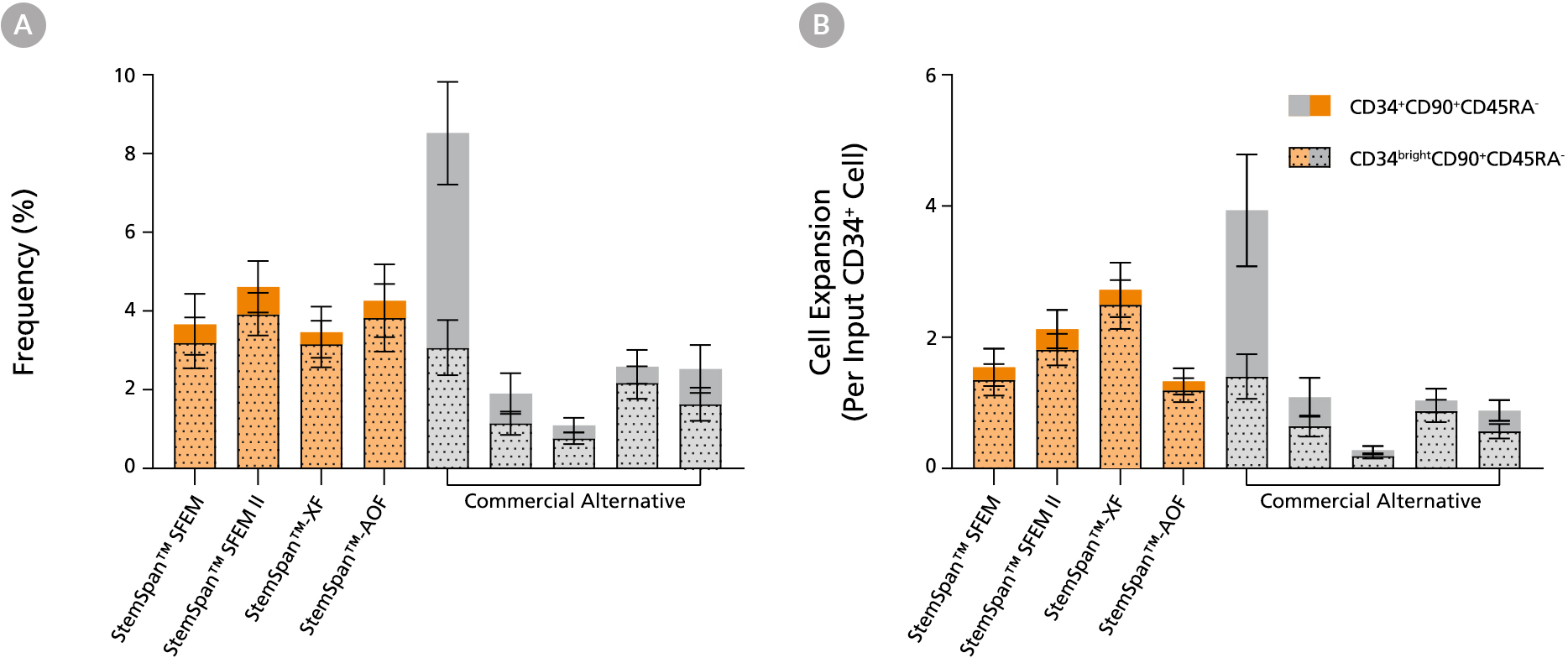
Figure 3. StemSpan™ Media Support Equal or Greater Expansion of Primitive Human CD34brightCD90+CD45RA- Cells Than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in select StemSpan™ media (StemSpan™ SFEM, StemSpan™ SFEM II, StemSpan™-XF, or StemSpan™-AOF, orange bars), and in five xeno-free media formulations from other suppliers (Commercial Alternative, grey bars) including (in random order) CTS StemPro HSC (Thermo), SCGM (Cellgenix), X-VIVO 15 (Lonza), Stemline II (Sigma), and StemPro 34 (Thermo). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of CD34+CD90+CD45RA- (solid) and CD34brightCD90+CD45RA-(dotted overlay) cells in culture were based on viable cell counts and flow cytometry results as shown in Figure 1. StemSpan™ media showed similar or significantly higher expansion of CD34brightCD90+CD45RA- cells (P < 0.05 compared to five media from other suppliers, calculated using one-way ANOVA followed by Dunnett’s post hoc test) and StemSpan™-AOF, the only animal origin-free formulation tested, showed equivalent performance to all xeno-free competitors tested. Data shown are mean ± SEM (n = 8).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version StemSpan™-ACF (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version, StemSpan™-AOF (Catalog #100-0130) was comparable.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
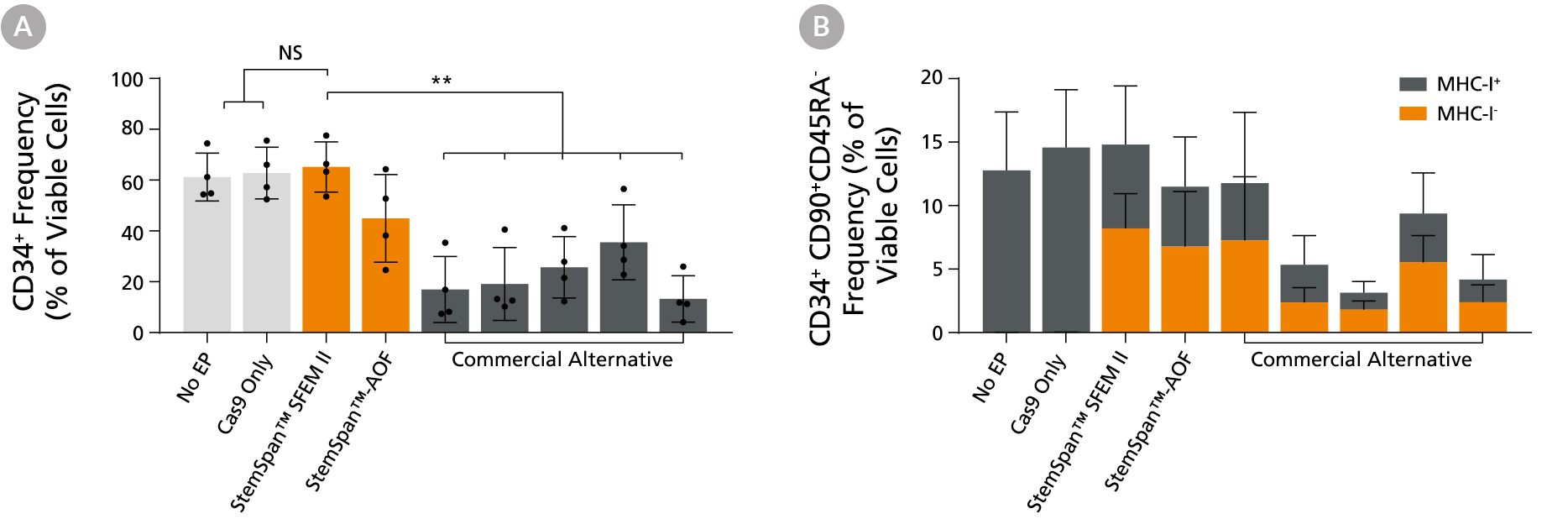
Figure 4. StemSpan™ Media Support Better CD34+ and Primitive CD34+CD90+CD45RA- HSPC Expansion in a Genome Editing Application Compared with Alternative Commercial Media
Purified CB-derived CD34+ cells were cultured for 2 days in select StemSpan™ media (StemSpan™ SFEM II or StemSpan™-AOF, orange bars), or five xeno-free media formulations from other suppliers (gray bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. Cells were then electroporated using Arcitect™ CRISPR-Cas9 RNP complexes containing crRNA:tracrRNA targeting beta-2-microglobulin (B2M), and cultured for an additional 4 days in the same conditions. Knockout efficiency as measured by staining for MHC-I and analyzing by flow cytometry, was similar in all media tested, ~70-80%. (A) The percentage of CD34+ cells and (B) CD34+CD90+CD45RA- cells were quantified by flow cytometry 4 days post-electroporation. Data shown are mean + SD (n = 4 donors; **P < 0.01).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF (Catalog #100-0130) was comparable.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1 μM. For more information including data comparing UM171 and UM729, see Fares et al., 2014.

 网站首页
网站首页