概要
The Starter Kit for MethoCult™ H4034 Optimum (MethoCult™ GF H4034) is recommended for laboratories in the initial stages of establishing procedures for assessing human hematopoietic progenitor cells using colony-forming unit (CFU) assays. These products support the growth of clonogenic hematopoietic progenitor cells from human bone marrow, peripheral blood, cord blood, leukapheresis products and purified progenitor-enriched cells. H4034 supports the growth of erythroid progenitors (BFU-E and CFU-E), granulocyte-macrophage progenitors (CFU-GM, CFU-G and CFU-M) and multi-potential granulocyte, erythroid, macrophage, megakaryocyte progenitors (CFU-GEMM). Each kit contains instructional materials in addition to all reagents and materials necessary to perform 24 duplicate assays.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
技术资料
数据及文献
Data
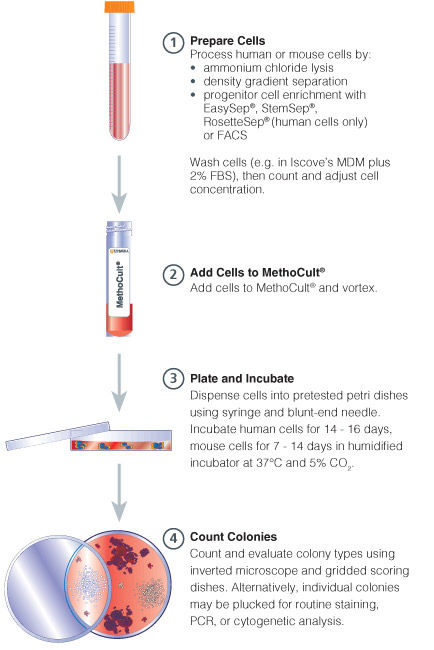
Figure 1. Procedure Summary for Hematopoietic CFU Assays
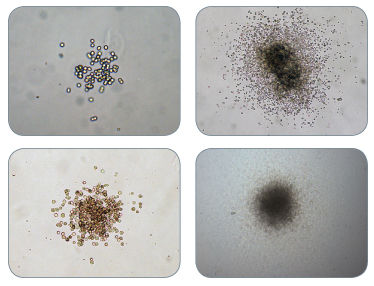
Figure 2. Examples of Colonies Derived from CFU-GM in MethoCult™ H4034 Optimum
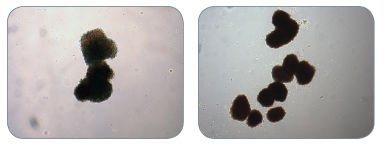
Figure 3. Examples of Colonies Derived from BFU-E in MethoCult™ H4034 Optimum
Publications (9)
Blood 2003 JAN
Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells.
Abstract
Abstract
We developed a new human stromal cell line that could expand human hematopoietic progenitor/stem cells. Primary human bone marrow stromal cells were infected with retrovirus containing the human telomerase catalytic subunit (hTERT) gene, resulting in increased population doubling and the acquisition of cell immortalization. Characteristics of the hTERT-transduced stromal (hTERT-stromal) cells were identical with those of the primary stromal cells in terms of morphologic appearance and expression of surface antigens. Human cord blood (CB) CD34(+) cells were expanded by coculture with primary stromal or hTERT-stromal cells in the presence of stem cell factor, thrombopoietin, and Flk-2/Flt-3 ligand under serum-free condition. The degree of expansion of CD34(+) cells and total number of colony-forming units in culture (CFU-Cs) after 2 weeks' coculture with the hTERT-stromal cells were nearly the same as those after 2 weeks' coculture with primary stromal cells (CD34(+) cells, 118-fold +/- 8-fold versus 117-fold +/- 13-fold; CFU-Cs, 71-fold +/- 5-fold versus 67-fold +/- 5-fold of initial cell number). CB expansion on hTERT-stromal cells occurred at a similar rate through 7 weeks. In contrast, the rate of CB expansion on primary stromal cells had drastically declined at 7 weeks. In nonobese diabetic/severe combined immunodeficiency (SCID) mice, the degree of engraftment of SCID-repopulating cells that had been cocultured with hTERT-stromal cells for 4 weeks was significantly higher than that of precocultured CB cells. These results indicate that this hTERT-stromal cell line could be useful for ex vivo expansion of hematopoietic progenitor/stem cells and for analyzing the microenvironment of human bone marrow.
Stem cells (Dayton, Ohio) 2003 JAN
Differences between peripheral blood and cord blood in the kinetics of lineage-restricted hematopoietic cells: implications for delayed platelet recovery following cord blood transplantation.
Abstract
Abstract
Cord blood (CB) cells are a useful source of hematopoietic cells for transplantation. The hematopoietic activities of CB cells are different from those of bone marrow and peripheral blood (PB) cells. Platelet recovery is significantly slower after transplantation with CB cells than with cells from other sources. However, the cellular mechanisms underlying these differences have not been elucidated. We compared the surface marker expression profiles of PB and CB hematopoietic cells. We focused on two surface markers of hematopoietic cell immaturity, i.e., CD34 and AC133. In addition to differences in surface marker expression, the PB and CB cells showed nonidentical differentiation pathways from AC133(+)CD34(+) (immature) hematopoietic cells to terminally differentiated cells. The majority of the AC133(+)CD34(+) PB cells initially lost AC133 expression and eventually became AC133(-)CD34(-) cells. In contrast, the AC133(+)CD34(+) CB cells did not go through the intermediate AC133(-)CD34(+) stage and lost both markers simultaneously. Meanwhile, the vast majority of megakaryocyte progenitors were of the AC133(-)CD34(+) phenotype. We conclude that the delayed recovery of platelets after CB transplantation is due to both subpopulation distribution and the process of differentiation from AC133(+)CD34(+) cells.
Stem cells (Dayton, Ohio) 2000 JAN
Murine stromal cell line HESS-5 maintains reconstituting ability of Ex vivo-generated hematopoietic stem cells from human bone marrow and cytokine-mobilized peripheral blood.
Abstract
Abstract
Human bone marrow (BM) or mobilized peripheral blood (mPB) CD34(+) cells have been shown to loose their stem cell quality during culture period more easily than those from cord blood (CB). We previously reported that human umbilical CB stem cells could effectively be expanded in the presence of human recombinant cytokines and a newly established murine bone marrow stromal cell line HESS-5. In this study we assessed the efficacy of this xenogeneic coculture system using human BM and mPB CD34(+) cells as materials. We measured the generation of CD34(+)CD38(-) cells and colony-forming units, and assessed severe-combined immunodeficient mouse-repopulating cell (SRC) activity using cells five days after serum-free cytokine-containing culture in the presence or the absence of a direct contact with HESS-5 cells. As compared with the stroma-free culture, the xenogeneic coculture was significantly superior on expansion of CD34(+)CD38(-) cells and colony-forming cells and on maintenance of SRC activity. The PKH26 study demonstrated that cell division was promoted faster in cells cocultured with HESS-5 cells than in cells cultured without HESS-5 cells. These results indicate that HESS-5 supports rapid generation of primitive progenitor cells (PPC) and maintains reconstituting ability of newly generated stem cells during ex vivo culture irrespective of the source of samples. This xenogeneic coculture system will be useful for ex vivo manipulation such as gene transduction to promote cell division and the generation of PPC and to prevent loss of stem cell quality.
Blood 2000 JAN
High levels of lymphoid expression of enhanced green fluorescent protein in nonhuman primates transplanted with cytokine-mobilized peripheral blood CD34(+) cells.
Abstract
Abstract
We have used a murine retrovirus vector containing an enhanced green fluorescent protein complimentary DNA (EGFP cDNA) to dynamically follow vector-expressing cells in the peripheral blood (PB) of transplanted rhesus macaques. Cytokine mobilized CD34(+) cells were transduced with an amphotropic vector that expressed EGFP and a dihydrofolate reductase cDNA under control of the murine stem cell virus promoter. The transduction protocol used the CH-296 recombinant human fibronectin fragment and relatively high concentrations of the flt-3 ligand and stem cell factor. Following transplantation of the transduced cells, up to 55% EGFP-expressing granulocytes were obtained in the peripheral circulation during the early posttransplant period. This level of myeloid marking, however, decreased to 0.1% or lower within 2 weeks. In contrast, EGFP expression in PB lymphocytes rose from 2%-5% shortly following transplantation to 10% or greater by week 5. After 10 weeks, the level of expression in PB lymphocytes continued to remain at 3%-5% as measured by both flow cytometry and Southern blot analysis, and EGFP expression was observed in CD4(+), CD8(+), CD20(+), and CD16/56(+) lymphocyte subsets. EGFP expression was only transiently detected in red blood cells and platelets soon after transplantation. Such sustained levels of lymphocyte marking may be therapeutic in a number of human gene therapy applications that require targeting of the lymphoid compartment. The transient appearance of EGFP(+) myeloid cells suggests that transduction of a lineage-restricted myeloid progenitor capable of short-term engraftment was obtained with this protocol. (Blood. 2000;95:445-452)
Experimental hematology 1999 NOV
Comparison of in vitro drug-sensitivity of human granulocyte-macrophage progenitors from two different origins: umbilical cord blood and bone marrow.
Abstract
Abstract
Predictive in vitro hematotoxicity assays using human cells will provide estimation of tolerable level and aid considerably the development of agents with greater therapeutic activity and less toxicity. Human hematopoietic cells can be derived from three sources: human bone marrow by sternal or femoral aspiration, mobilized peripheral blood, or umbilical cord blood samples collected from placentas after deliveries. Because of the difficulties to have a continuous supply of bone marrow cells from normal human donors and the related ethical problems, we performed a study to compare the sensitivity of human bone marrow cells (h-BMC) and human cord blood cells (h-CBC) to chemicals in order to confirm if h-CBC can readily replace bone marrow cells in checking the sensitivity of GM-CFU progenitors to drugs as preliminarily reported in literature. Our results showed that the prediction of IC50 values in human model is quite similar by using h-BMC or h-CBC. On the contrary, the type of medium influenced in a significant way the ICs determination of some drugs.
Blood 1996 JAN
Rapid and efficient selection of human hematopoietic cells expressing murine heat-stable antigen as an indicator of retroviral-mediated gene transfer.
Abstract
Abstract
Recombinant retroviruses offer many advantages for the genetic modification of human hematopoietic cells, although their use in clinical protocols has thus far given disappointing results. There is therefore an important need to develop new strategies that will allow effectively transduced primitive hematopoietic target populations to be both rapidly characterized and isolated free of residual nontransduced but biologically equivalent cells. To address this need, we constructed a murine stem cell virus (MSCV)-based retroviral vector containing the 228-bp coding sequence of the murine heat-stable antigen (HSA) and generated helper virus-free amphotropic MSCV-HSA producer cells by transfection of GP-env AM12 packaging cells. Light density and, in some cases, lineage marker-negative (lin-) normal human marrow or mobilized peripheral blood cells preactivated by exposure to interleukin-3 (IL-3), IL-6, and Steel factor in vitro for 48 hours were then infected by cocultivation with these MSCV-HSA producer cells for a further 48 hours in the presence of the same cytokines. Fluorescence-activated cell sorting (FACS) analysis of the cells 24 hours later showed 21% to 41% (mean, 27%) of those that were still CD34+ to have acquired the ability to express HSA. The extent of gene transfer to erythroid and granulopoietic progenitors (burst-forming unit-erythroid and colony-forming unit-granulocyte-macrophage), as assessed by the ability of these cells to form colonies of mature progeny in the presence of normally toxic concentrations of G418, averaged 11% and 12%, respectively, in 6 experiments. These values could be increased to 100% and 77%, respectively, by prior isolation of the CD34+HSA+ cell fraction and were correspondingly decreased to an average of 2% and 5%, respectively, in the CD34+HSA- cells. In addition, the extent of gene transfer to long-term culture-initiating cells (LTC-IC) was assessed by G418 resistance. The average gene transfer to LTC-IC-derived colony-forming cells in the unsorted population was textless or = 7% in 4 experiments. FACS selection of the initially CD34+HSA+ cells increased this value to 86% and decreased it to 3% for the LTC-IC plated from the CD34+HSA- cells. Transfer of HSA gene expression to a phenotypically defined more primitive subpopulation of CD34+ cells, ie, those expressing little or no CD38, could also be shown by FACS analysis of infected populations 24 hours after infection. These findings underscore the potential use of retroviral vectors encoding HSA for the specific identification and non-toxic selection immediately after infection of retrovirally transduced populations of primitive human hematopoietic cells. In addition, such vectors should facilitate the subsequent tracking of their marked progeny using multiparameter flow cytometry.

 网站首页
网站首页