概要
STEMdiff™ Erythroid Kit has been optimized for differentiation of cells maintained in mTeSR™1 (Catalog #85850), mTeSR™ Plus (Catalog #100-0276), and TeSR™-E8™ (Catalog #05990).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
| Safety Data Sheet 1 | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
| Safety Data Sheet 2 | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
| Safety Data Sheet 3 | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
| Safety Data Sheet 4 | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
| Safety Data Sheet 5 | STEMdiff™ Erythroid Kit | 100-0074 | All | English |
数据及文献
Data
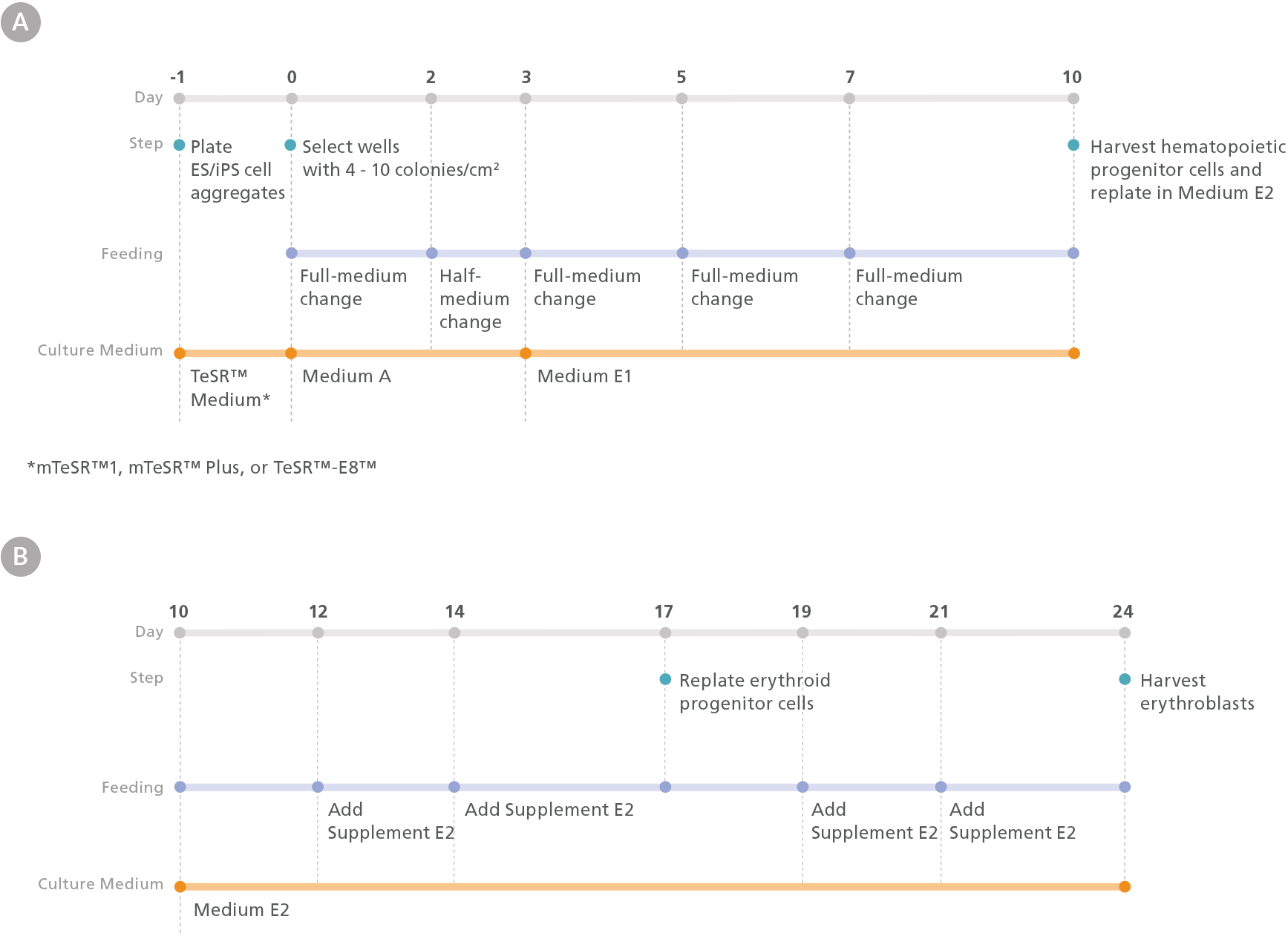
Figure 1. Erythroid Differentiation Protocol
The protocol involves two main steps: (A) hematopoietic specification of human embryonic stem (hES) or induced pluripotent stem (hiPS) cells and (B) differentiation of hES or hiPS cell-derived HPCs into erythroid cells. One day prior to differentiation, hES or hiPS cells were plated as small aggregates (100 - 200 µm diameter) at a density of 15 - 20 aggregates/cm2 in mTeSR™1, mTeSR™ Plus, or TeSR™-E8™ medium on Corning® Matrigel®-coated plates. After attaching overnight, mesoderm differentiation was initiated by changing the medium to Medium A (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Hematopoietic Supplement A). On day 3, the medium was changed to Medium E1 (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Erythroid Supplement E1) to induce hematoendothelial and hematopoietic specification. On day 10, hematopoietic progenitor cells (HPCs) in suspension were harvested from the culture and counted. Following this, HPCs were replated in Medium E2 (StemSpan™ SFEM II + STEMdiff™ Erythroid Supplement E2) at a density of 4 x 10 4 cells/mL and cultured for 14 days to generate erythroblasts.
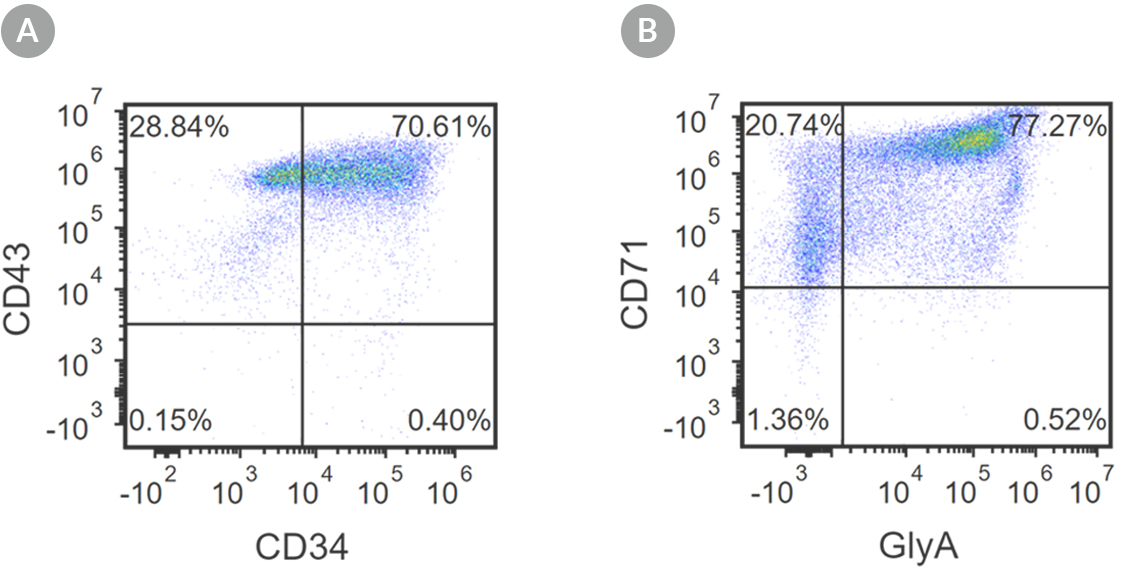
Figure 2. Robust and Efficient Generation of CD71+GlyA+ Erythroids Using STEMdiff™ Erythroid Kit
hES and hiPS cells were induced to differentiate into CD71+GlyA+ cells using the 24-day protocol shown in Figure 1. Following the initial 10-day culture period and at the end of the 24-day protocol, cells were harvested from the supernatant and analyzed by flow cytometry. Dead cells were excluded by light scatter profile and propidium iodide staining. (A) Representative flow cytometry plot for iPSC-derived (1C) cells analyzed on day 10. Cells were identified as CD34+CD43+ hematopoietic progenitors. (B) After 24 days of culture, cells were identified as having differentiated into CD71+ GlyA+ erythroblasts. The erythroid identity of the generated cells was additionally assessed by cell morphology and hemoglobin expression (see Figure 4).
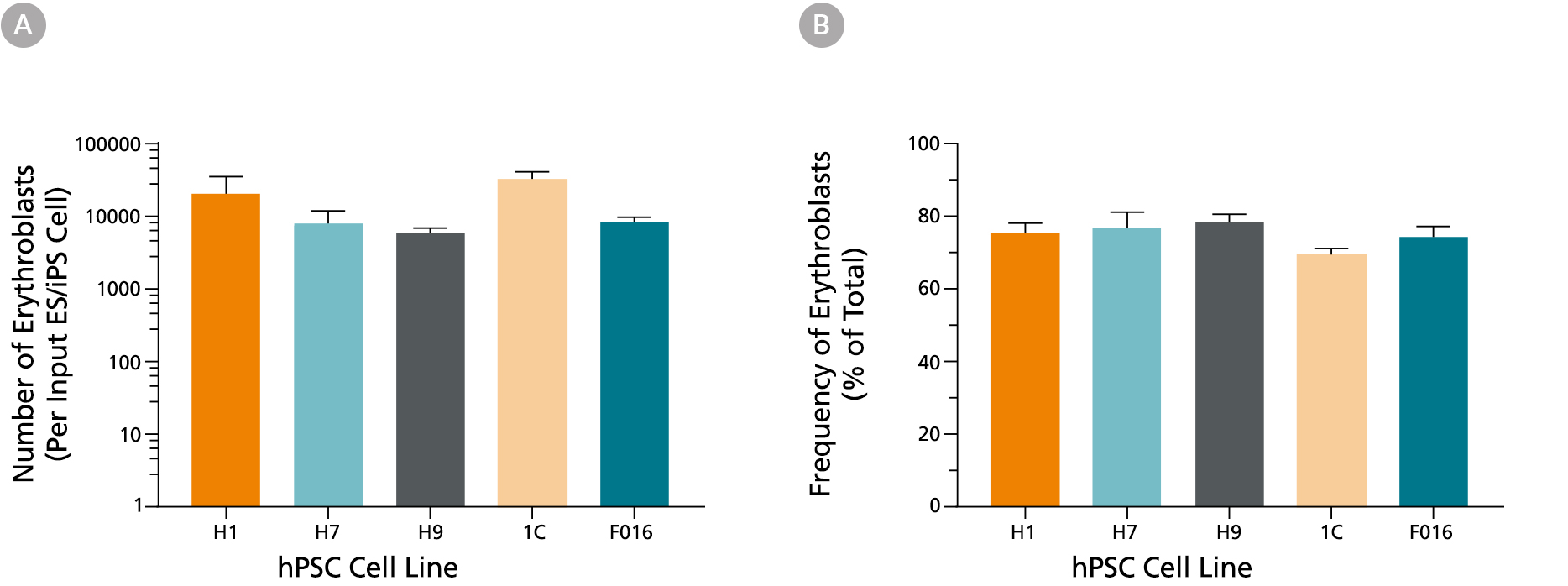
Figure 3. Production of Erythroid Cells from hES and hiPS cells After 24 Days of Culture in the STEMdiff™ Erythroid Kit
(A) Shown are the average numbers of viable, GlyA+ erythroid cells generated per input cell after culturing hES and hiPS cells for 24 days using the STEMdiff™ Erythroid Kit. (B) The overall frequency of GlyA+ erythroid cells present in culture after 24 days is shown as a percentage of total nucleated cells present. Data are presented as mean and SEM (n = 5 - 21) for six individual hES and hiPS cell lines.

Figure 4. hES and hiPS cells-Derived Erythroid Cells Are Hemoglobinized and Display Typical Erythroid Morphology
(A) Erythroid cells generated with the STEMdiff™ Erythroid Kit express a mix of primitive (embryonic) and definitive (fetal, adult) hemoglobin. Shown are the results of qPCR analysis for globin gene expression after 24 days of culture. (B) A picture of the cell pellet shows that cells produced in culture are hemoglobinized. (C) Cells display typical basophilic erythroblast morphology after 24 days of culture using the STEMdiff™ Erythroid Kit (40X magnification, May-Grunwald Giemsa stain).
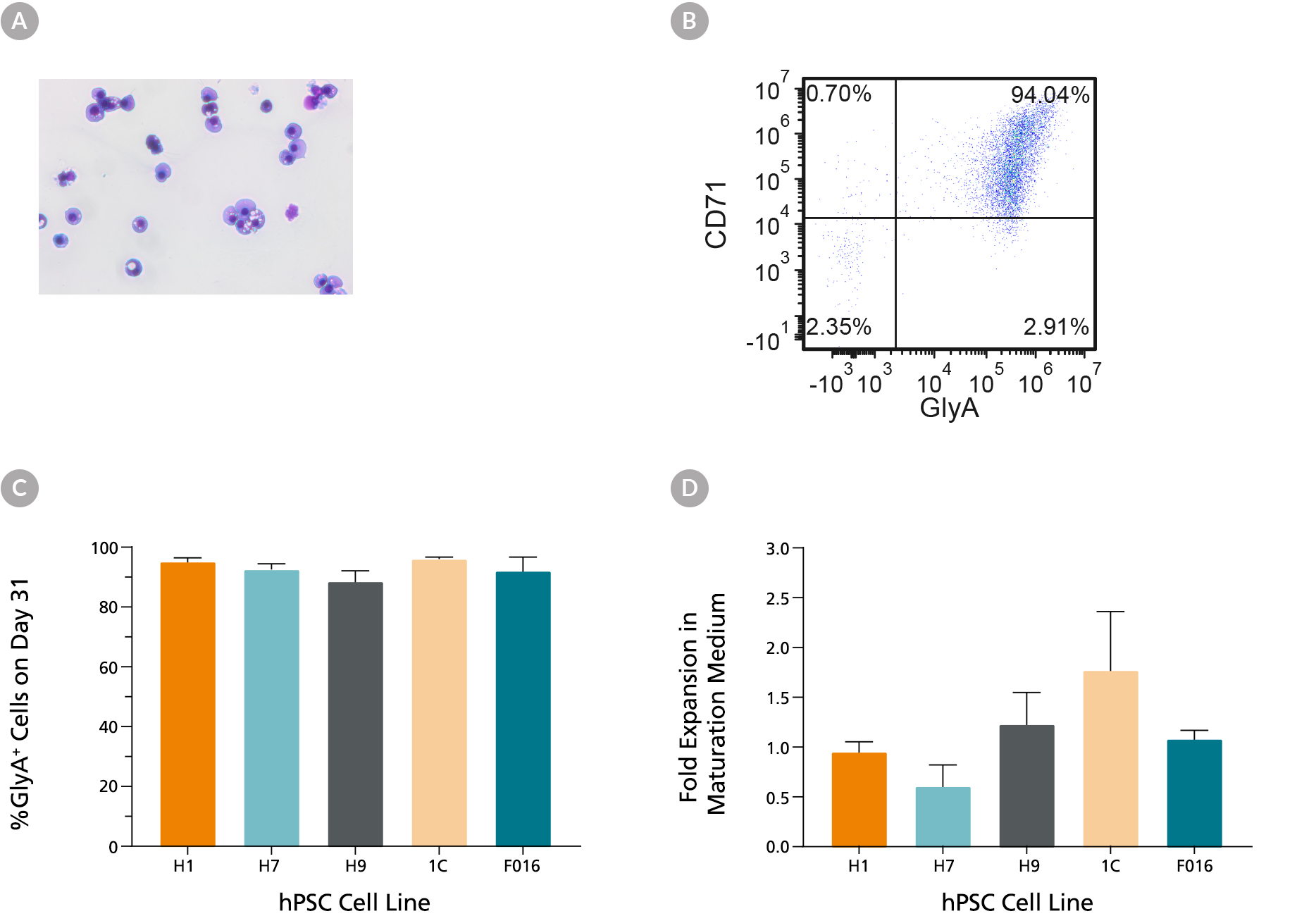
Figure 5. hES and hiPS Cell-Derived Erythroid Cells Can Mature into CD71low/GlyA+ Normoblasts
hES and hiPS cell-derived erythroblasts can mature further when cultured for an additional 7 days in maturation conditions (StemSpan™ SFEM II medium with human serum (3%) and EPO (3 U/mL)). (A) Further maturation of the erythroid cells results in smaller cells with condensed nuclei, which is typical for orthochromatic normoblasts (40X magnification, May-Grunwald Giemsa stain). (B) A representative flow cytometry plot shows that over 90% of the cells are GlyA+ and majority of cells have decreased CD71 expression, which is consistent with erythroid maturation. (C) Bar graphs summarize the average frequencies of GlyA+ erythroid cells on day 31 across all 6 hiPS cell lines. (D) Notably, fold expansion data shows that cell numbers are maintained during the maturation culture. Data are presented as mean and SEM (n=2-6).

 网站首页
网站首页