概要
技术资料
数据及文献
Data
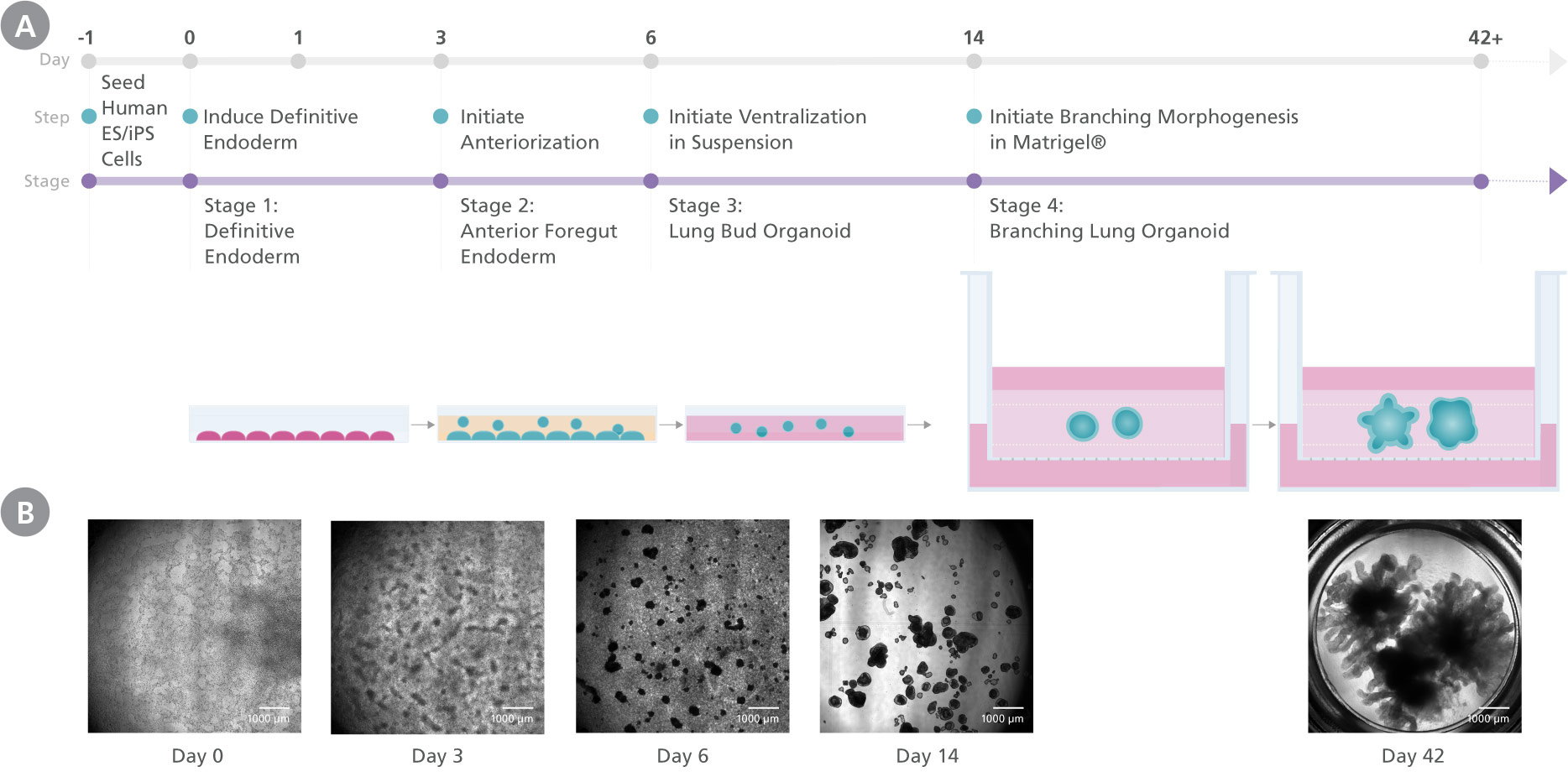
Figure 1. Generation Of Branching Lung Organoids Using the STEMdiff™ Branching Lung Organoid Kit
(A) Human PSC cultures progress through a four-stage differentiation process to generate human branching lung organoids. By the end of stage 1 (day 3), cultures exhibit characteristics typical of definitive endoderm and anterior foregut differentiation is initiated. During stage 2 (days 3 - 6) anterior foregut endoderm buds are released from the monolayer, and are then suspended to form ventralized lung bud organoids in stage 3 (days 6 - 14). In stage 4, the lung bud organoids are embedded into Matrigel sandwich cultures to mature into branching lung organoids. (B) Morphological representation of the culture at different stages.
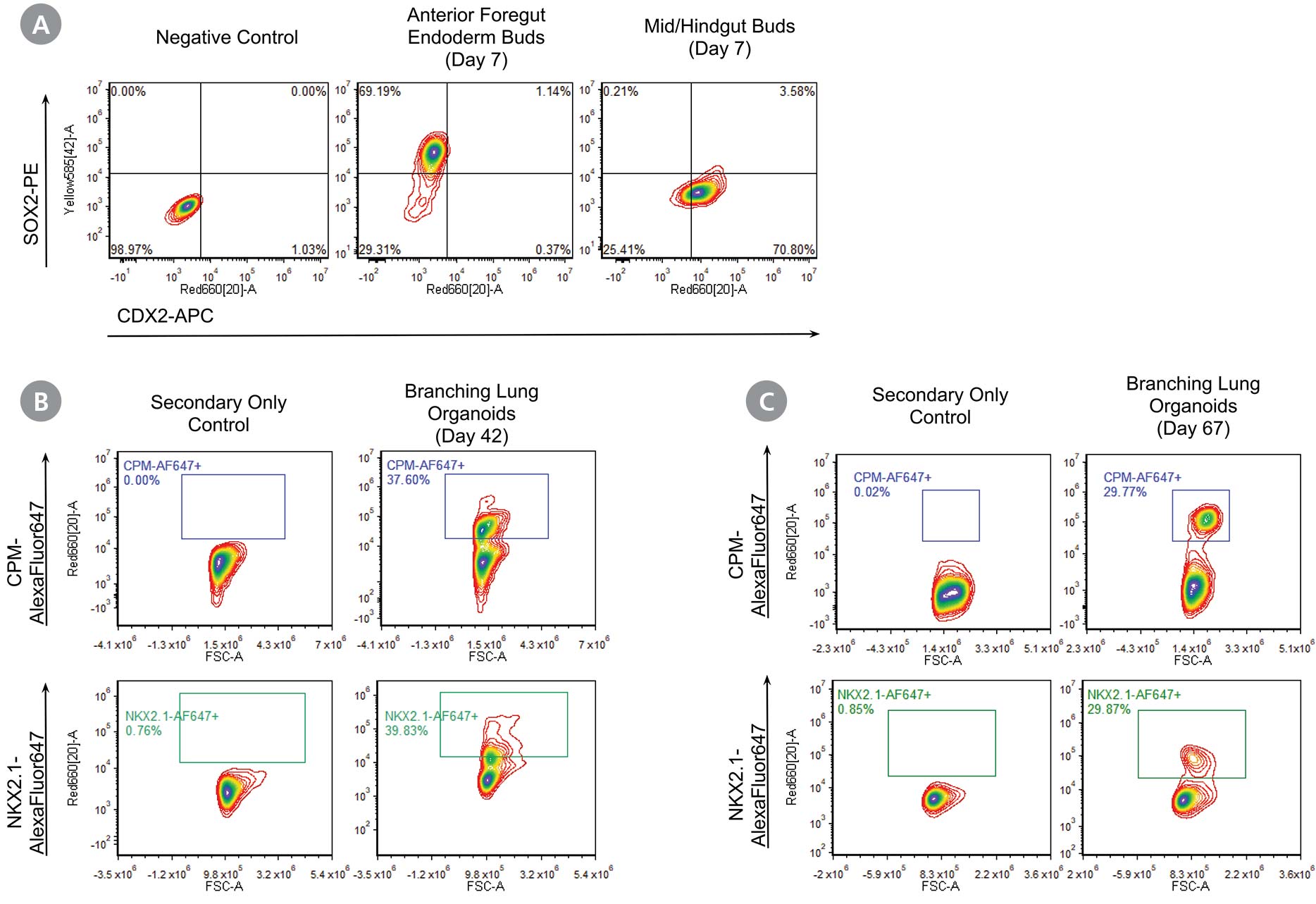
Figure 2. STEMdiff™ Branching Lung Organoid Kit Supports Expression of Key Lung Markers at Different Stages
Flow cytometric analysis at different stages of the STEMdiff™ Branching Lung Organoid Kit workflow shows protein expression of key lung markers. (A) Anterior foregut endoderm (AFE) buds generated at the end of stage 2 demonstrates high expression of the anterior foregut marker, SOX2 and absence of mid/hindgut marker, CDX2. (B) Cells from branching lung organoids on day 42 (stage 4) express lung progenitor marker NKX2.1 and its surrogate cell surface marker CPM. (C) NKX2.1 and CPM expression are maintained in long-term cultures as seen by flow cytometric analysis of cells from branching lung organoids (day 67).
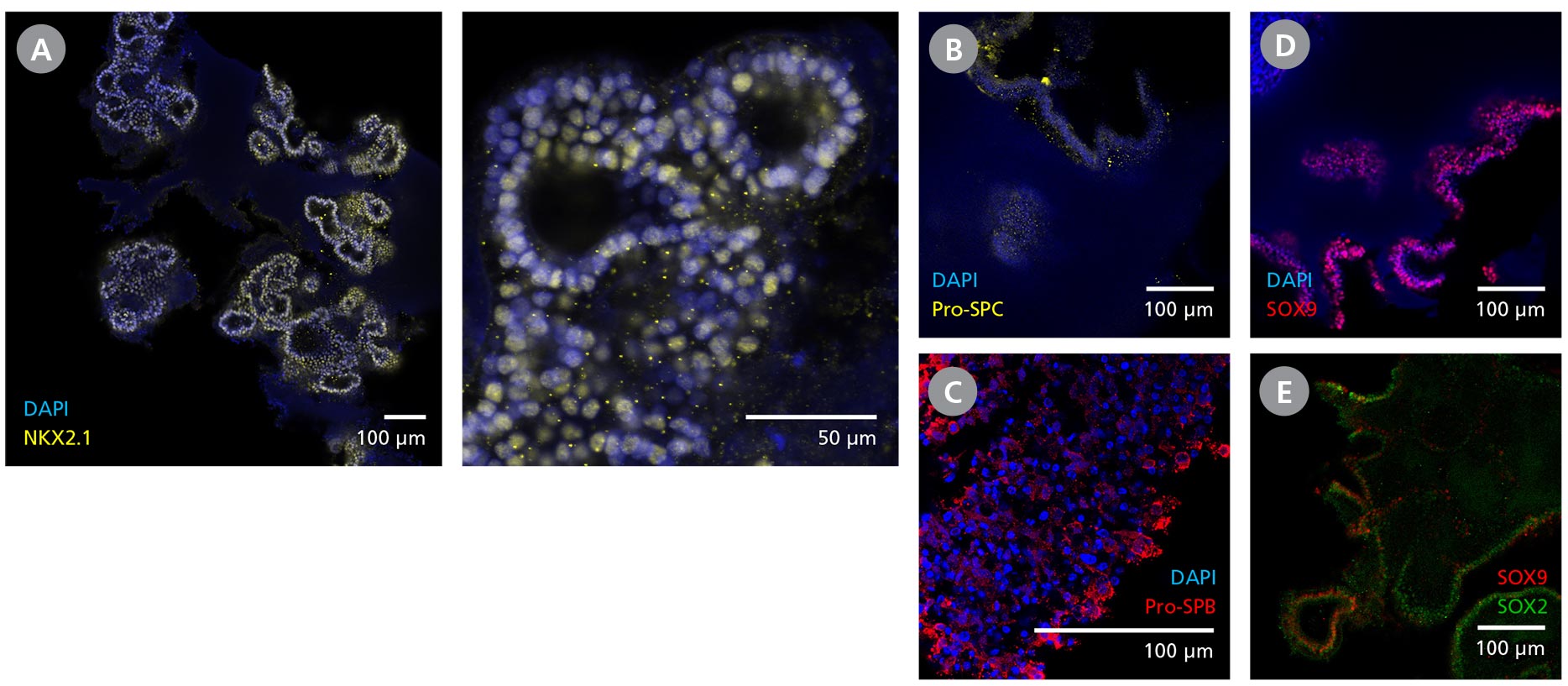
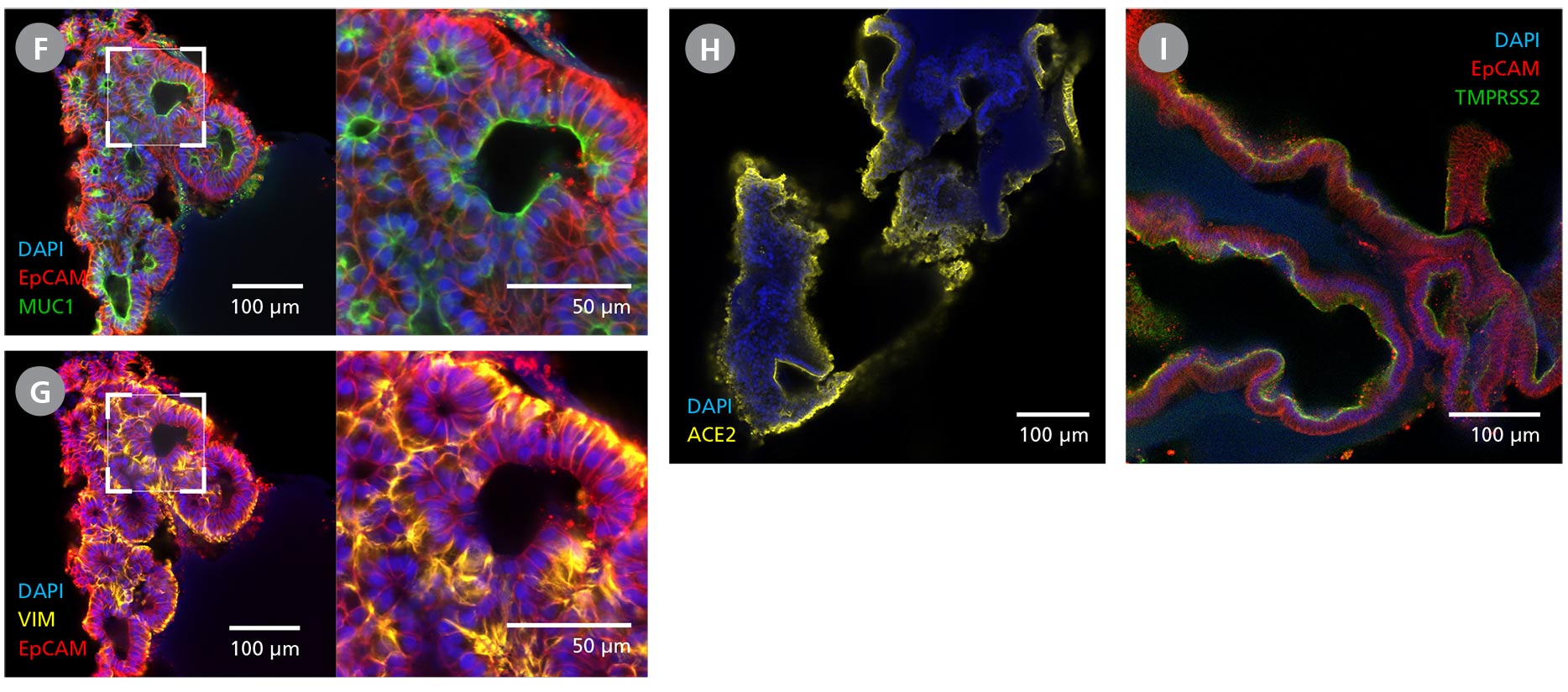
Figure 3. Branching Lung Organoids Cultured in STEMdiff™ Branching Lung Organoid Kit Feature Key Protein Markers and Exhibit Branching Morphogenesis
(A) Branching lung organoids express lung progenitor marker NKX2.1 throughout their branching structures, and (B, C) demonstrate the presence of alveolar type II-like cells with Pro-surfactant protein B and C expressions. (D, E) These organoids undergo proximodistal differentiation demonstrated by the differential expression of SOX2 and SOX9. (F) MUC1 can be found luminally expressed while the (G) organoids are surrounded by VIM-expressing mesenchyme. (H, I) Branching lung organoids generated in STEMdiff™ Branching Lung Organoid Kit also express proteins associated with SARS-CoV-2 entry, ACE2 and TMPRSS2. Protein expression was visualized by immunohistochemistry and confocal microscopy of branching lung organoids on day 63.
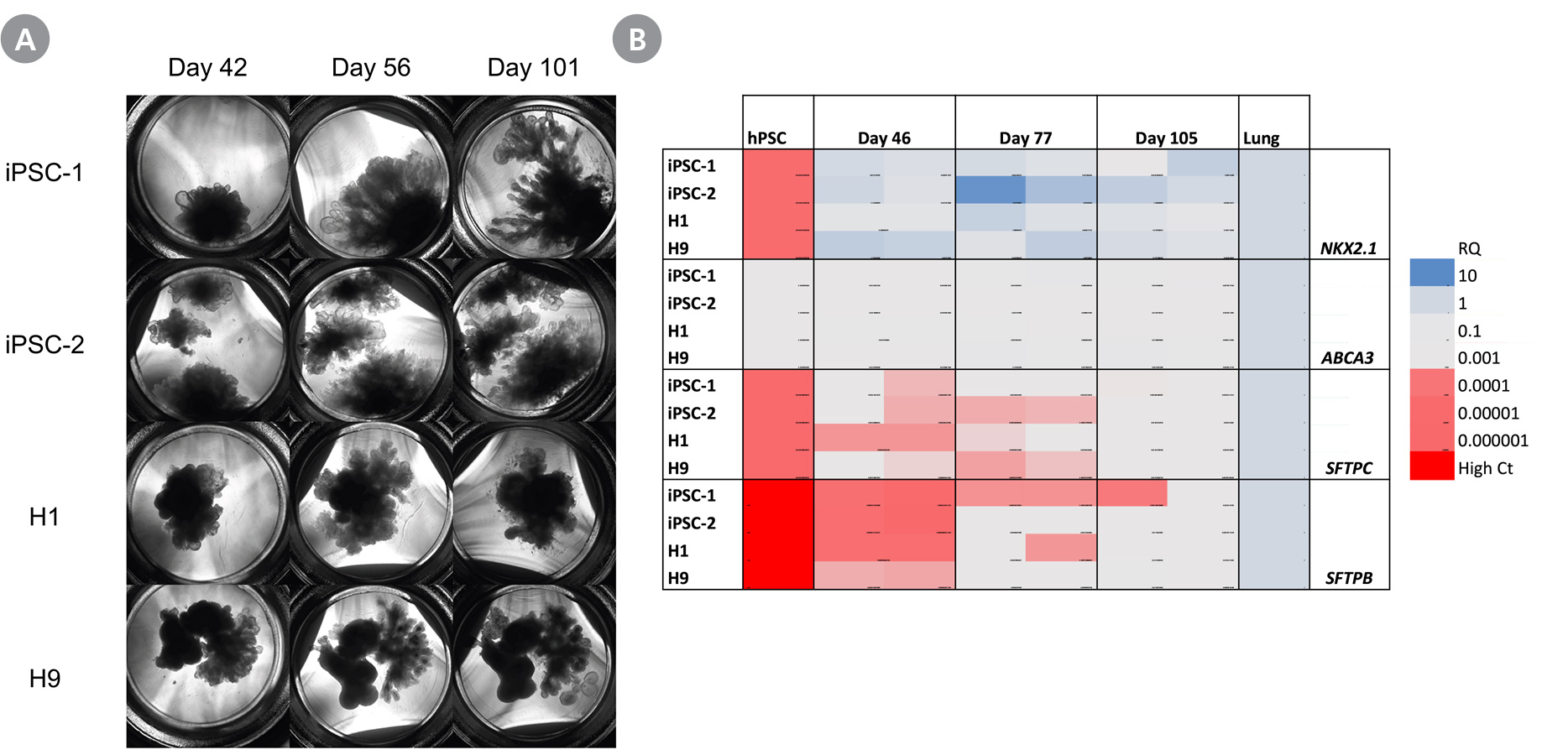
Figure 4. Branching Lung Organoids Cultured in STEMdiff™ Branching Lung Organoid Maturation Kit For Extended Periods Express More Mature Lung Markers
(A) The branching tips of branching lung organoids derived from peripheral blood endothelial progenitor cell-derived iPS cells (iPSC-1), peripheral blood mononuclear cell-derived iPS cells (iPSC-2) or embryonic stem cells (H1 and H9) continue to grow and branch up to day 101. (B) The expression levels of more mature distal lung markers ABCA3, SFTPC, and SFTPB increase overtime. Morphology and gene expression of branching lung organoids cultured up to day 105 were assessed by RT-qPCR. RQ-values were normalized to TBP and compared to commercially available lung RNA.
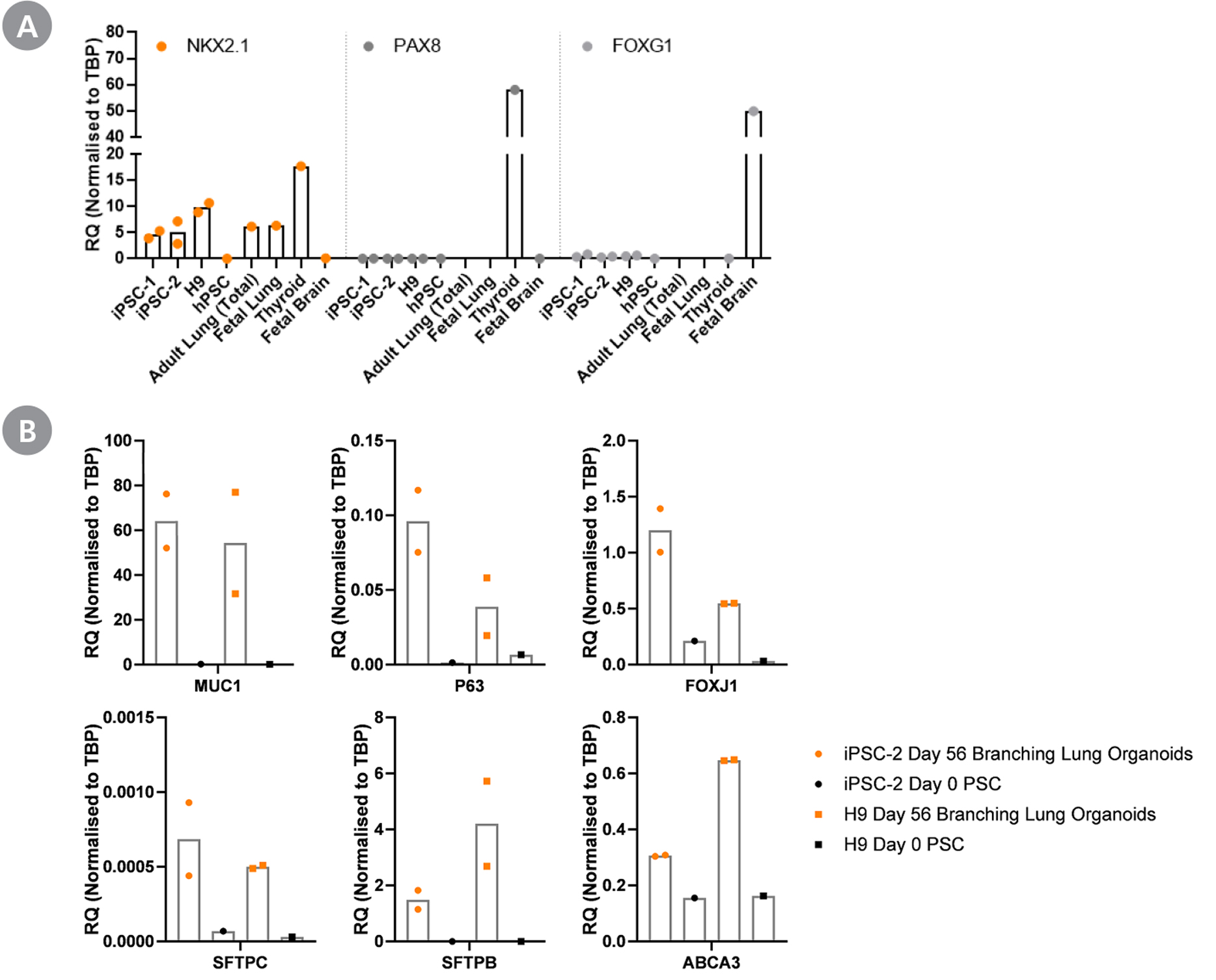
Figure 5. Branching Lung Organoid Cells Are Specifically Differentiated Towards the Lung
Gene expression profile of branching lung organoids (day 42+) analysed by RT-qPCR show presence of key lung markers and absence of off-target markers. Branching lung organoids at day 42 express (A) lung-thyroid-forebrain marker NKX2.1 and does not exhibit thyroid marker PAX8 and forebrain marker FOXG1. (B) The expressions of proximal lung markers MUC1, P63, and FOXJ1 and distal lung markers SFTPC, SFTPB, and ABCA3 are upregulated in day 56 branching lung organoids, when compared to respective pluripotent stem cells (PSCs). All expression levels are normalized to the housekeeping gene TBP. iPSC-1: peripheral blood endothelial progenitor cell-derived iPS cells; iPSC-2: peripheral blood mononuclear cell-derived iPS cells.

 网站首页
网站首页