概要
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | STEMdiff™ Astrocyte Differentiation Kit | 100-0013 | All | English |
| Special Protocol | STEMdiff™ Astrocyte Differentiation Kit | 100-0013 | All | English |
| Safety Data Sheet 1 | STEMdiff™ Astrocyte Differentiation Kit | 100-0013 | All | English |
| Safety Data Sheet 2 | STEMdiff™ Astrocyte Differentiation Kit | 100-0013 | All | English |
数据及文献
Data
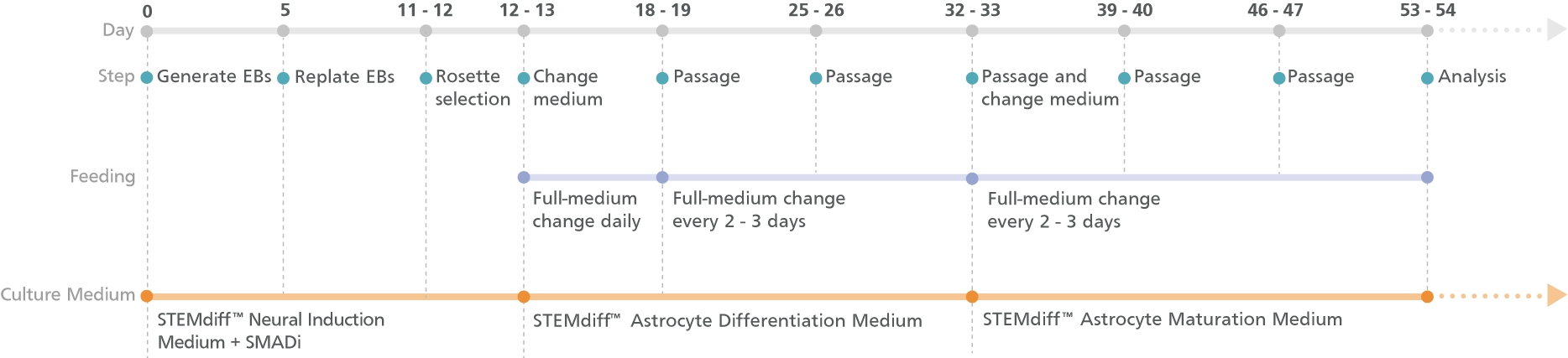
Figure 1. Schematic for the Embryoid Body Protocol
Cortical-type astrocyte precursors can be generated in 20 days from hPSC-derived neural progenitor cells (NPCs) after selecting neural rosettes from replated embryoid bodies. For the maturation of precursors to cortical-type astrocytes, see the PIS.
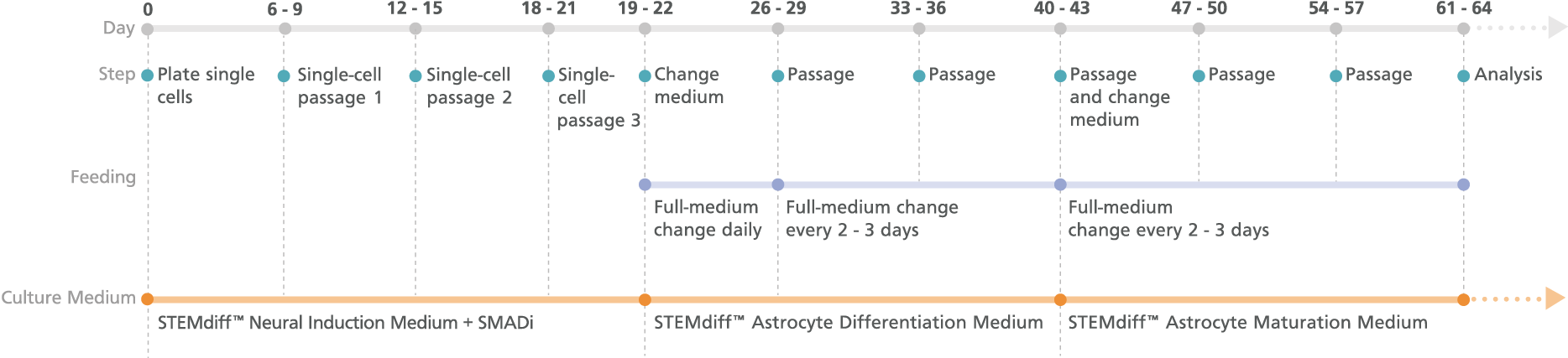
Figure 2. Schematic for the Monolayer Protocol
Cortical-type astrocyte precursors can be generated in 21 days from neural progenitor cell (NPC) monolayers derived from embryonic and induced pluripotent stem cells after three single-cell passages. For the maturation of precursors to cortical-type astrocytes, see the PIS.
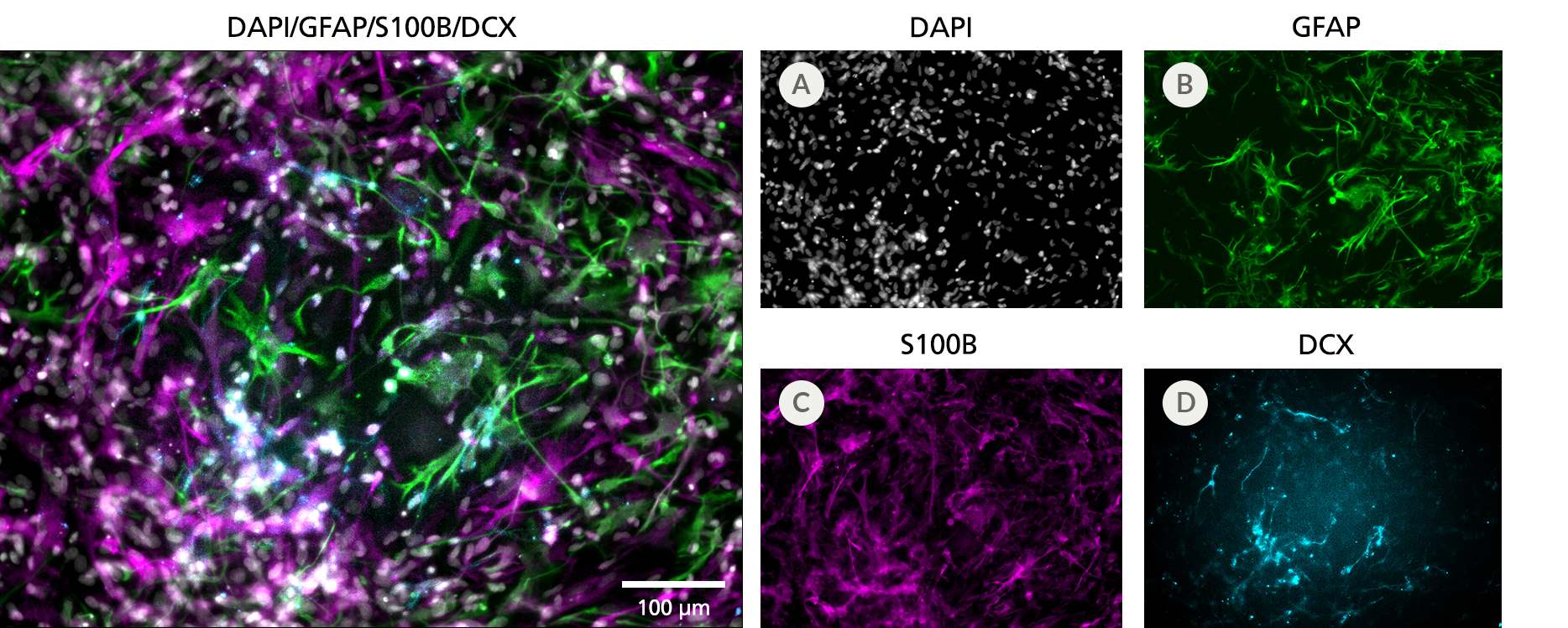
Figure 3. Cortical-Type Astrocytes Are Generated After Culture in STEMdiff™ Astrocyte Differentiation and Maturation Kits
NPCs generated from hPSCs in TeSR™-E8™ using the STEMdiff™ SMADi Neural Induction Kit embryoid body (EB) protocol were differentiated and matured to cortical-type astrocytes using the STEMdiff™ Astrocyte Differentiation and Maturation Kits. Cortical-type astrocytes were formed after iPS cell-derived NPCs were cultured with the STEMdiff™ Astrocyte Differentiation Kit for 3 weeks and STEMdiff™ Astrocyte Maturation Kit for 3 weeks. (A) Nuclei are labeled with DAPI (gray). The resulting cultures contain a highly pure population of astrocytes, which are (B) more than 60% GFAP-positive (green) and (C) more than 70% S100B-positive (magenta), with (D) fewer than 15% neurons (DCX-positive cells, cyan). Scale bar = 100 μm.
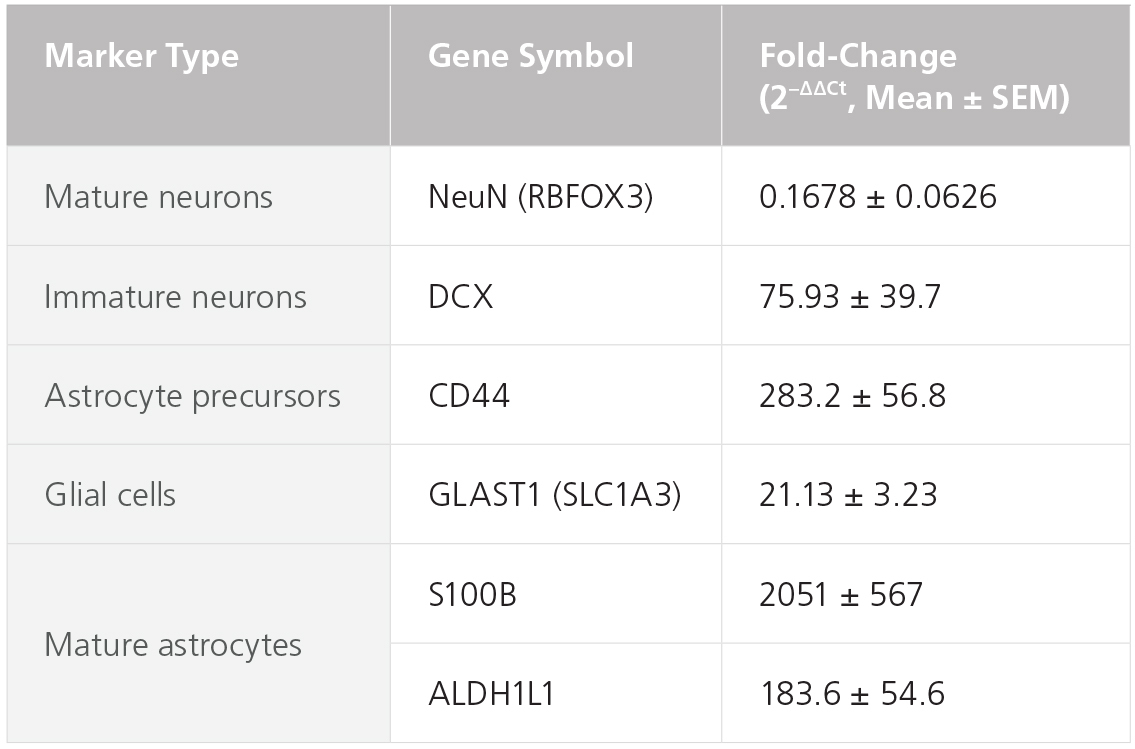
Figure 4. STEMdiff™ Astrocyte Kits Generate Cells Expressing Expected Levels of Genes Characteristic for Astrocytes
Embryonic stem and induced pluripotent stem cells from a variety of lines (n = 6, maintained in mTeSR™1 or TeSR™-E8™) were differentiated to NPCs using the STEMdiff™ SMADi Neural Induction Kit embryoid body protocol. Cells were then grown in STEMdiff™ Astrocyte Differentiation Kit for 3 weeks followed by STEMdiff™ Astrocyte Maturation Kit for 3 weeks prior to analysis. Expression levels were measured by quantitative PCR (qPCR) and normalized to hPSC controls relative to housekeeping genes 18S and TBP.
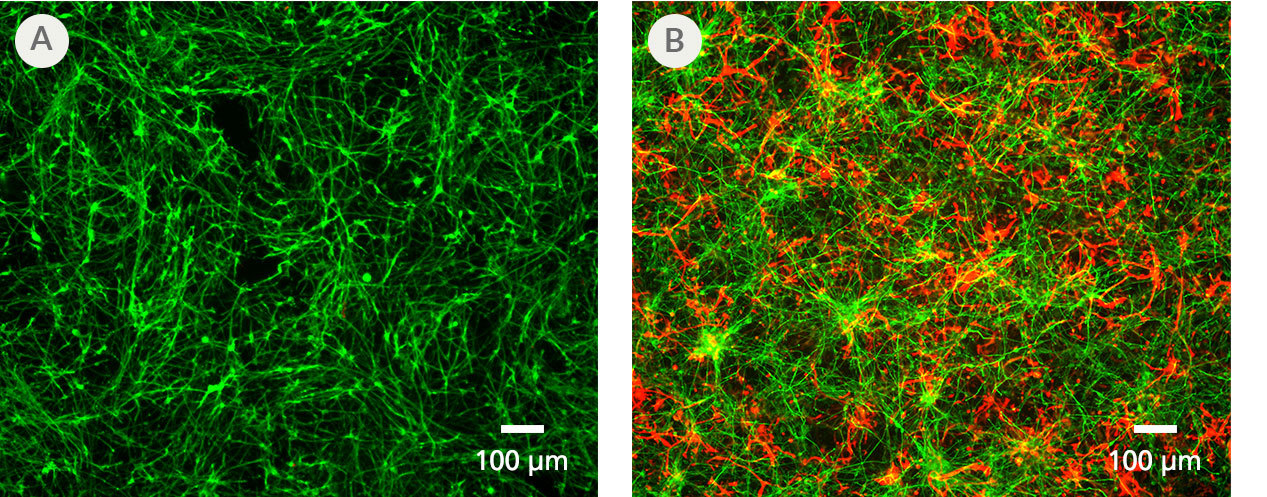
Figure 5. PSC-Derived Astrocytes and Neurons Can Be Co-Cultured to Model Cell-Cell Interactions In Vitro
NPCs generated from the H1 cell line were differentiated to astrocytes using STEMdiff™ Astrocyte Differentiation and Maturation Kits. H9 cell-derived NPCs were differentiated to forebrain-type neurons using STEMdiff™ Forebrain Neuron Differentiation and Maturation Kits. For co-culture, matured astrocytes were seeded onto forebrain neurons that had been in STEMdiff™ Forebrain Neuron Maturation Medium for at least one week. Co-cultures were then switched to STEMdiff™ Forebrain Neuron Maturation Medium the following day and for the remaining co-culture. (A) Neurons cultured alone, following the co-culture feeding schedule, are labeled with DCX (green). (B) DCX-positive neurons (green) and astrocytes (GFAP, red) can be co-cultured for at least 1 - 2 weeks prior to analysis. For a detailed co-culture protocol, please see the Methods Library.

Figure 6. PSC-Derived Neurons Survive and Mature when Co-Cultured with PSC-Derived Astrocytes
NPCs generated from the STiPS-R038 cell line were differentiated to astrocytes using STEMdiff™ Astrocyte Differentiation and Maturation Kits. STiPS-M001 cell-derived NPCs were differentiated to forebrain-type neurons using STEMdiff™ Forebrain Neuron Differentiation and Maturation Kits. After co-culture for one week, neurons (A) had significantly increased neurite outgrowth as measured on MAP2-positive neurons with the NeuriteTracer plugin for ImageJ (M Pool et al. J Neurosci Methods, 2008) and (B) were more numerous than neurons cultured alone using the same feeding schedule. *, p < 0.05; **, p < 0.01.

 网站首页
网站首页