概要
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | ReproRNA™-OKSGM Kit | 05930 | All | English |
| Product Information Sheet | ReproRNA™-OKSGM | 05931 | All | English |
| Safety Data Sheet 1 | ReproRNA™-OKSGM Kit | 05930 | All | English |
| Safety Data Sheet 2 | ReproRNA™-OKSGM Kit | 05930 | All | English |
| Safety Data Sheet 3 | ReproRNA™-OKSGM Kit | 05930 | All | English |
| Safety Data Sheet 4 | ReproRNA™-OKSGM Kit | 05930 | All | English |
| Safety Data Sheet | ReproRNA™-OKSGM | 05931 | All | English |
数据及文献
Data

Figure 1. Schematic of ReproRNA™-OKSGM, a Single‑Stranded RNA Replicon Vector
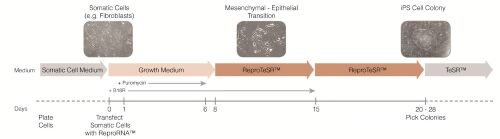
Figure 2. Schematic of Reprogramming Timeline with ReproRNA™-OKSGM
Somatic cells are transfected with ReproRNA™-OKSGM at day 0, and cultured in Advanced DMEM (AdvDMEM) with puromycin. After 6 days of puromycin selection post-transfection, cells are cultured in ReproTeSR™ for the remainder of the reprogramming induction phase until iPS cell colonies emerge. B18R recombinant protein is also added during the first 2 weeks after transfection to inhibit the interferon response and increase cell viability. Typically, by day 20, iPS cell colonies are large enough to be isolated and propagated in TeSR™ media. *TeSR™ = TeSR™ family media (mTeSR™1, TeSR™2, TeSR™-E8™).

Figure 3. ReproRNA™-OKSGM Vector Efficiently Reprograms Fibroblasts
Dermal fibroblasts were transfected with the ReproRNA™-OKSGM vector and reprogrammed under feeder-dependent (standard KOSR-containing hES cell medium on irradiated mouse embryonic fibroblasts (iMEFs)) or feeder-independent conditions (ReproTeSR™ on Corning® Matrigel®). Fibroblasts (passage 4) were reprogrammed with average efficiencies of 0.10 ± 0.03% (hES cell medium) and 0.20 ± 0.01% (ReproTeSR™). Reprogramming efficiency of fibroblasts with ReproRNA™ and ReproTeSR™ is comparable to that reported with Sendai virus.¹ (n ≥ 6; Data shown are mean ± SEM).

Figure 4. Feeder-Free Reprogramming with ReproRNA™-OKSGM Vector and ReproTeSR™ Generates iPS Cell Colonies with Superior Colony Morphology
Representative images of iPS cell colonies were generated using ReproRNA™‑OKSGM and cultured in (A) standard hES cell medium on irradiated mouse embryonic fibroblasts (iMEFs) or (B) ReproTeSR™ on Corning® Matrigel®. iPS cell colonies derived using ReproTeSR™ exhibit more defined borders, compact morphology, and reduced differentiation as compared to the ES cell medium.

Figure 5. Human iPS Cells Generated with ReproRNA™-OKSGM Express Undifferentiated Cell Markers
Human iPS cells generated with ReproRNA™-OKSGM display high expression of undifferentiated cell markers (OCT4 and TRA-1-60) as shown by flow cytometry analysis after 12 passages in mTeSR™1. (Filled histogram = sample, hollow histogram = secondary antibody only).

Figure 6. iPS Cells Derived Using ReproRNA™-OKSGM Display a Normal Karyotype
Karyogram of iPS cells derived with ReproRNA™-OKSGM and cultured in mTeSR™1 for 8 passages shows that a normal karyotype is retained.

Figure 7. ReproRNA™-OKSGM Derived iPS Cells Have the Capacity to Differentiate to Cells of the Three Germ Layers
Human iPS cells derived with ReproRNA™-OKSGM and maintained in mTeSR™1 for 7 passages were differentiated into cells of the three germ layers. Endoderm specification was achieved using the STEMdiff™ Definitive Endoderm Kit and flow cytometry analysis shows a high percentage of cells (98.7%) positive for endoderm markers (CXCR4+SOX17+). Mesoderm induction was achieved with STEMdiff™ Mesoderm Induction Medium as shown by the high percentage of cells (98.6%) expressing Brachyury (T). Ectoderm specification was demonstrated using STEMdiff™ Neural Induction Medium. CNS-enriched NPC cultures expressing PAX6 (green) and stained with DAPI (blue) are shown.

 网站首页
网站首页