概要
PneumaCult™-Ex Plus and either PneumaCult™-ALI or PneumaCult™-ALI-S constitute a fully integrated BPE-free culture system for in vitro human airway modeling. This robust and defined system is a valuable tool for basic respiratory research, toxicity studies, and drug development.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | PneumaCult™-Ex Plus Medium | 05040 | All | English |
| Safety Data Sheet 1 | PneumaCult™-Ex Plus Medium | 05040 | All | English |
| Safety Data Sheet 2 | PneumaCult™-Ex Plus Medium | 05040 | All | English |
数据及文献
Data

Figure 1. Overview of the PneumaCult™ culture system
Expansion of human bronchial epithelial cells (HBECs) in submerged culture is performed with PneumaCult™-Ex Plus or PneumaCult™-Ex. During the early “Expansion Phase” of the air-liquid interface (ALI) culture procedure, PneumaCult™-Ex Plus or PneumaCult™-Ex is applied to the apical and basal chambers. Upon reaching confluence, the culture is air-lifted by removing the culture medium from both chambers, and adding PneumaCult™-ALI to the basal chamber only. Differentiation into a pseudostratified mucociliary epithelium is obtained following 21-28 days of incubation and can be maintained for more than one year.

Figure 2. HBECs cultured in PneumaCult™-Ex Plus have a faster expansion rate compared to those cultured in PneumaCult™-Ex and Bronchial Epithelial Growth Media
Commercially available, cryopreserved P1 HBECs were seeded into PneumaCult™-Ex Plus, PneumaCult™-Ex, or Bronchial Epithelial Growth Media. Cells cultured in PneumaCult™-Ex Plus have a significantly higher proliferation rate over 9 passages compared to those maintained in either control medium (n=6).

Figure 3. Representative morphology of HBECs
Representative live culture images for P4 HBECs cultured in PneumaCult™-Ex Plus, PneumaCult™-Ex, or Bronchial Epithelial Growth Media. Cells cultured in PneumaCult™-Ex Plus (A) are smaller and more tightly packed than those cultured in PneumaCult™-Ex (B) or Bronchial Epithelial Growth Media (C). All images were taken using a 10X objective.
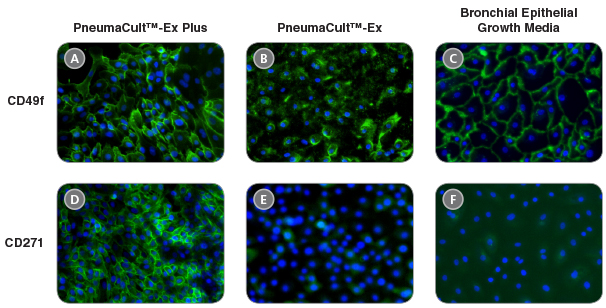
Figure 4. HBECs cultured in PneumaCult™-Ex Plus maintain widespread expression of the basal cell markers CD49f and CD271
Immunocytochemistry detection of basal cell markers - CD49f (A, B, and C) and CD271 (D, E, and F) - for P4 HBECs cultured in PneumaCult™-Ex Plus (A and D), PneumaCult™-Ex (B and E), and Bronchial Epithelial Growth Media (C and F). All images were taken using a 10X objective.

Figure 5. HBECs cultured in PneumaCult™-Ex Plus have a higher proportion of CD271+CD49f+ cells
P4 HBECs cultured in PneumaCult™-Ex Plus (A), PneumaCult™-Ex (B), and Bronchial Epithelial Growth Media (C) were characterized by flow cytometry to detect expression of the basal cell markers CD49f and CD271. HBECs cultured in PneumaCult™-Ex Plus (A) have a higher proportion of cells coexpressing CD49f and CD271, compared to those cultured in PneumaCult™-Ex (B) and Bronchial Epithelial Growth Media (C).

Figure 6. HBECs cultured in PneumaCult™-Ex Plus differentiate into a pseudostratified mucociliary epithelium at later passages with the use of PneumaCult™-ALI
P4 HBECs were seeded and passaged using PneumaCult™-Ex Plus, PneumaCult™-Ex, or Bronchial Epithelial Growth Media, followed by ALI differentiation at each passage (P5-8) with the use of PneumaCult™-ALI. The ALI cultures at 28 days post air-lift were fixed and stained with antibodies for cilia marker AC-tubulin (red) and the goblet cell marker Muc5AC (green). The nuclei are counterstained with DAPI (blue). All images were taken using a 20X objective.
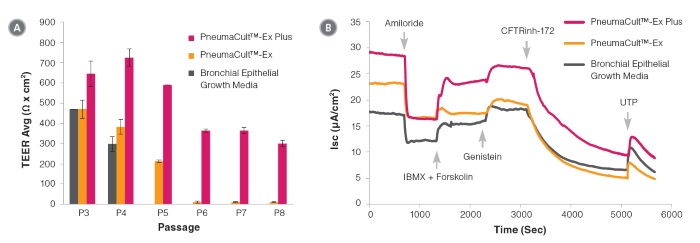
Figure 7. Electrophysiological characterization of differentiated HBECs (P4) that were expanded in PneumaCult™-Ex Plus, PneumaCult™-Ex, and Bronchial Epithelial Growth Media
Transepithelial electrical resistance (TEER) (A) and representative characterization of the ion channel activities (B) for ALI cultures at 28 days post air-lift using HBECs expanded in PneumaCult™-Ex Plus, PneumaCult™-Ex, or Bronchial Epithelial Growth Media. Amiloride: ENaC inhibitor. IBMX and Forskolin: CFTR activators. Genistein: CFTR potentiator. CFTRinh-172: CFTR inhibitor. UTP: Calciumactivated Chloride channels (CaCCs) activator. All ALI differentiation cultures were performed using PneumaCult™-ALI.

 网站首页
网站首页