概要
PneumaCult™-Ex is a defined, serum- and BPE-free cell culture medium that supports rapid expansion of human airway epithelial cells.
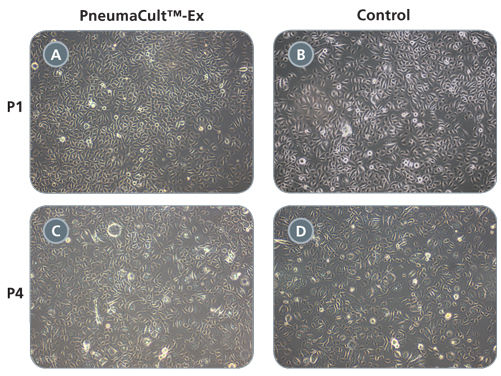
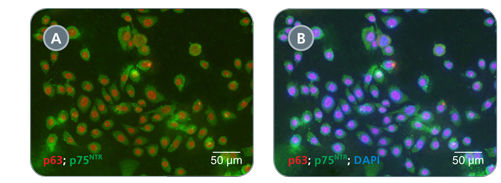
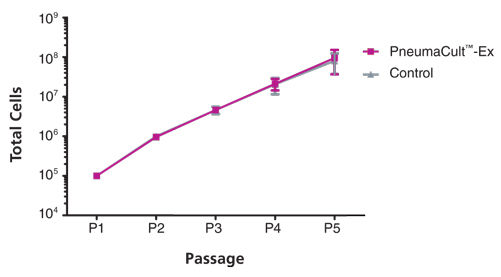
Primary airway epithelial cells cultured in PneumaCult™-Ex expand rapidly over at least 3 passages while maintaining a cobblestone morphology and uniform expression of the basal cell markers p63 and p75NTR. Additionally, cells cultured in PneumaCult™-Ex can be differentiated to form a pseudostratified mucociliary epithelium when cultured at the air-liquid interface in PneumaCult™-ALI.
Together, PneumaCult™-Ex and PneumaCult™-ALI constitute a fully integrated BPE-free culture system for in vitro human airway modeling. This robust and defined system is a valuable tool for basic respiratory research, toxicity studies, and drug development.
数据及文献
Publications (14)
International journal of molecular sciences 2020 sep
Impact of KLF4 on Cell Proliferation and Epithelial Differentiation in the Context of Cystic Fibrosis.
L. Sousa et al.
Abstract
Cystic fibrosis (CF) cells display a more cancer-like phenotype vs. non-CF cells. KLF4 overexpression has been described in CF and this transcriptional factor acts as a negative regulator of wt-CFTR. KLF4 is described as exerting its effects in a cell-context-dependent fashion, but it is generally considered a major regulator of proliferation, differentiation, and wound healing, all the processes that are also altered in CF. Therefore, it is relevant to characterize the differential role of KLF4 in these processes in CF vs. non-CF cells. To this end, we used wt- and F508del-CFTR CFBE cells and their respective KLF4 knockout (KO) counterparts to evaluate processes like cell proliferation, polarization, and wound healing, as well as to compare the expression of several epithelial differentiation markers. Our data indicate no major impact of KLF4 KO in proliferation and a differential impact of KLF4 KO in transepithelial electrical resistance (TEER) acquisition and wound healing in wt- vs. F508del-CFTR cells. In parallel, we also observed a differential impact on the levels of some differentiation markers and epithelial-mesencymal transition (EMT)-associated transcription factors. In conclusion, KLF4 impacts TEER acquisition, wound healing, and the expression of differentiation markers in a way that is partially dependent on the CFTR-status of the cell.
Cells 2020 oct
Aprotinin Inhibits SARS-CoV-2 Replication.
D. Bojkova et al.
Abstract
Severe acute respiratory syndrome virus 2 (SARS-CoV-2) is the cause of the current coronavirus disease 19 (COVID-19) pandemic. Protease inhibitors are under consideration as virus entry inhibitors that prevent the cleavage of the coronavirus spike (S) protein by cellular proteases. Herein, we showed that the protease inhibitor aprotinin (but not the protease inhibitor SERPINA1/alpha-1 antitrypsin) inhibited SARS-CoV-2 replication in therapeutically achievable concentrations. An analysis of proteomics and translatome data indicated that SARS-CoV-2 replication is associated with a downregulation of host cell protease inhibitors. Hence, aprotinin may compensate for downregulated host cell proteases during later virus replication cycles. Aprotinin displayed anti-SARS-CoV-2 activity in different cell types (Caco2, Calu-3, and primary bronchial epithelial cell air-liquid interface cultures) and against four virus isolates. In conclusion, therapeutic aprotinin concentrations exert anti-SARS-CoV-2 activity. An approved aprotinin aerosol may have potential for the early local control of SARS-CoV-2 replication and the prevention of COVID-19 progression to a severe, systemic disease.
The Journal of biological chemistry 2020 nov
Loss of versican and production of hyaluronan in lung epithelial cells are associated with airway inflammation during RSV infection.
G. G. Kellar et al.
Abstract
Airway inflammation is a critical feature of lower respiratory tract infections caused by viruses such as respiratory syncytial virus (RSV). A growing body of literature has demonstrated the importance of extracellular matrix (ECM) changes such as the accumulation of hyaluronan (HA) and versican in the subepithelial space in promoting airway inflammation; however, whether these factors contribute to airway inflammation during RSV infection remains unknown. To test the hypothesis that RSV infection promotes inflammation via altered HA and versican production, we studied an ex vivo human bronchial epithelial cell (BEC)/human lung fibroblast (HLF) co-culture model. RSV infection of BEC/HLF co-cultures led to decreased hyaluronidase expression by HLFs, increased accumulation of HA, and enhanced adhesion of U937 cells as would be expected with increased HA. HLF production of versican was not altered following RSV infection; however, BEC production of versican was significantly downregulated following RSV infection. In vivo studies with epithelial-specific versican-deficient mice [SPC-Cre(+) Vcan-/-] demonstrated that RSV infection led to increased HA accumulation compared to control mice which also coincided with decreased hyaluronidase expression in the lung. SPC-Cre(+) Vcan-/- mice demonstrated enhanced recruitment of monocytes and neutrophils in bronchoalveolar lavage fluid and increased neutrophils in the lung compared to SPC-Cre(-) RSV-infected littermates. Taken together, these data demonstrate that altered ECM accumulation of HA occurs following RSV infection and may contribute to airway inflammation. Additionally, loss of epithelial expression of versican promotes airway inflammation during RSV infection further demonstrating that versican's role in inflammatory regulation is complex and dependent on the microenvironment.
The Journal of biological chemistry 2020 nov
Genomic determinants implicated in the glucocorticoid-mediated induction of KLF9 in pulmonary epithelial cells.
M. M. Mostafa et al.
Abstract
Ligand-activated glucocorticoid receptor (GR) elicits variable glucocorticoid-modulated transcriptomes in different cell types. However, some genes, including Kr{\{u}}ppel-like factor 9 (KLF9) a putative transcriptional repressor demonstrate conserved responses. We show that glucocorticoids induce KLF9 expression in the human airways in vivo and in differentiated human bronchial epithelial (HBE) cells grown at air-liquid interface (ALI). In A549 and BEAS-2B pulmonary epithelial cells glucocorticoids induce KLF9 expression with similar kinetics to primary HBE cells in submersion culture. A549 and BEAS-2B ChIP-seq data reveal four common glucocorticoid-induced GR binding sites (GBSs). Two GBSs mapped to the 5'-proximal region relative to KLF9 transcription start site (TSS) and two occurred at distal sites. These were all confirmed in primary HBE cells. Global run-on (GRO)-sequencing indicated robust enhancer RNA (eRNA) production from three of these GBSs in BEAS-2B cells. This was confirmed in A549 cells plus submersion and ALI culture of HBE cells. Cloning each GBS into luciferase reporters revealed glucocorticoid-induced activity requiring a glucocorticoid response element (GRE) within each distal GBS. While the proximal GBSs drove modest reporter induction by glucocorticoids this region exhibited basal eRNA production RNA polymerase II enrichment and looping to the TSS plausibly underlying constitutive KLF9 expression. Post-glucocorticoid treatment interactions between distal and proximal GBSs and the TSS correlated with KLF9 induction. CBP/P300 silencing reduced proximal GBS activity but negligibly effected KLF9 expression. Overall a model for glucocorticoid-mediated regulation of KLF9 involving multiple GBSs is depicted. This work unequivocally demonstrates that mechanistic insights gained from cell-lines can translate to physiologically relevant systems."
Nature communications 2020 dec
MCL-1 gains occur with high frequency in lung adenocarcinoma and can be targeted therapeutically.
E. Munkhbaatar et al.
Abstract
Evasion of programmed cell death represents a critical form of oncogene addiction in cancer cells. Understanding the molecular mechanisms underpinning cancer cell survival despite the oncogenic stress could provide a molecular basis for potential therapeutic interventions. Here we explore the role of pro-survival genes in cancer cell integrity during clonal evolution in non-small cell lung cancer (NSCLC). We identify gains of MCL-1 at high frequency in multiple independent NSCLC cohorts, occurring both clonally and subclonally. Clonal loss of functional TP53 is significantly associated with subclonal gains of MCL-1. In mice, tumour progression is delayed upon pharmacologic or genetic inhibition of MCL-1. These findings reveal that MCL-1 gains occur with high frequency in lung adenocarcinoma and can be targeted therapeutically.
International journal of cancer 2018 JUL
Conditionally reprogrammed cells (CRC) methodology does not allow the in vitro expansion of patient-derived primary and metastatic lung cancer cells.
G. Sette et al.
Abstract
Availability of tumor and non-tumor patient-derived models would promote the development of more effective therapeutics for non-small cell lung cancer (NSCLC). Recently, conditionally reprogrammed cells (CRC) methodology demonstrated exceptional potential for the expansion of epithelial cells from patient tissues. However, the possibility to expand patient-derived lung cancer cells using CRC protocols is controversial. Here, we used CRC approach to expand cells from non-tumoral and tumor biopsies of patients with primary or metastatic NSCLC as well as pulmonary metastases of colorectal or breast cancers. CRC cultures were obtained from both tumor and non-malignant tissues with extraordinary high efficiency. Tumor cells were tracked in vitro through tumorigenicity assay, monitoring of tumor-specific genetic alterations and marker expression. Cultures were composed of EpCAM+ lung epithelial cells lacking tumorigenic potential. NSCLC biopsies-derived cultures rapidly lost patient-specific genetic mutations or tumor antigens. Similarly, pulmonary metastases of colon or breast cancer generated CRC cultures of lung epithelial cells. All CRC cultures examined displayed epithelial lung stem cell phenotype and function. In contrast, brain metastatic lung cancer biopsies failed to generate CRC cultures. In conclusion, patient-derived primary and metastatic lung cancer cells were negatively selected under CRC conditions, limiting the expansion to non-malignant lung epithelial stem cells from either tumor or non-tumor tissue sources. Thus, CRC approach cannot be applied for direct therapeutic testing of patient lung tumor cells, as the tumor-derived CRC cultures are composed of (non-tumoral) airway basal cells.
View All Publications

 网站首页
网站首页