概要
NeuroCult™ NS-A Proliferation Kit (Human) is a standardized, serum-free basal medium and supplement for the culture of human neural stem and progenitor cells from normal tissues or tumor samples, in the neurosphere or adherent monolayer system. When supplemented with appropriate cytokines, NeuroCult™ NS-A Proliferation Kit (Human) is optimized to maintain human neural stem cells in culture for extended periods of time without the loss of their self-renewal, proliferation, or differentiation potential.
NOTE: Addition of rh EGF (Catalog #78006), rh bFGF (Catalog #78003) and heparin (Catalog #07980) is required.
NOTE: Addition of rh EGF (Catalog #78006), rh bFGF (Catalog #78003) and heparin (Catalog #07980) is required.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | NeuroCult™ NS-A Proliferation Kit (Human) | 05751 | All | English |
| Special Protocol | NeuroCult™ NS-A Proliferation Kit (Human) | 05751 | All | English |
| Manual | NeuroCult™ NS-A Proliferation Kit (Human) | 05751 | All | English |
| Safety Data Sheet 1 | NeuroCult™ NS-A Proliferation Kit (Human) | 05751 | All | English |
| Safety Data Sheet 2 | NeuroCult™ NS-A Proliferation Kit (Human) | 05751 | All | English |
数据及文献
Data
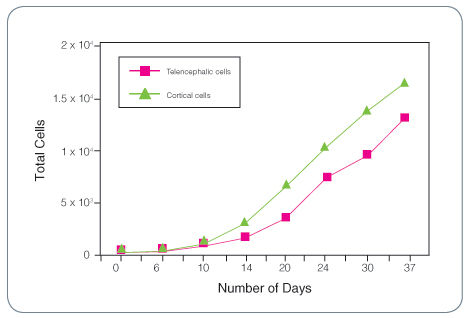
Figure 1. Total Cell Expansion for Fetal Human Telencephalic and Cortical Cells Cultured as Neurospheres with Complete NeuroCult™ Proliferation Medium (Human) Containing rh EGF, rh bFGF and Heparin
Publications (112)
Nature communications 2020 jul
Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy.
Abstract
Abstract
Cancer stem cells are critical for cancer initiation, development, and treatment resistance. Our understanding of these processes, and how they relate to glioblastoma heterogeneity, is limited. To overcome these limitations, we performed single-cell RNA sequencing on 53586 adult glioblastoma cells and 22637 normal human fetal brain cells, and compared the lineage hierarchy of the developing human brain to the transcriptome of cancer cells. We find a conserved neural tri-lineage cancer hierarchy centered around glial progenitor-like cells. We also find that this progenitor population contains the majority of the cancer's cycling cells, and, using RNA velocity, is often the originator of the other cell types. Finally, we show that this hierarchal map can be used to identify therapeutic targets specific to progenitor cancer stem cells. Our analyses show that normal brain development reconciles glioblastoma development, suggests a possible origin for glioblastoma hierarchy, and helps to identify cancer stem cell-specific targets.
Scientific Reports 2019 dec
BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma
Abstract
Abstract
Despite advances in therapy, glioblastoma remains an incurable disease with a dismal prognosis. Recent studies have implicated cancer stem cells within glioblastoma (glioma stem cells, GSCs) as mediators of therapeutic resistance and tumor progression. In this study, we investigated the role of the transforming growth factor-$\beta$ (TGF-$\beta$) superfamily, which has been found to play an integral role in the maintenance of stem cell homeostasis within multiple stem cell systems, as a mediator of stem-like cells in glioblastoma. We find that BMP and TGF-$\beta$ signaling define divergent molecular and functional identities in glioblastoma, and mark relatively quiescent and proliferative GSCs, respectively. Treatment of GSCs with BMP inhibits cell proliferation, but does not abrogate their stem-ness, as measured by self-renewal and tumorigencity. Further, BMP pathway activation confers relative resistance to radiation and temozolomide chemotherapy. Our findings define a quiescent cancer stem cell population in glioblastoma that may be a cellular reservoir for tumor recurrence following cytotoxic therapy.
Pediatric surgery international 2019 dec
A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury.
Abstract
Abstract
AIM OF THE STUDY Human breast milk reduces the risk and severity of necrotizing enterocolitis (NEC). Exosomes are extracellular vesicles (EVs) found in high concentrations in milk, and they mediate intercellular communication and immune responses. The aim of this study is to compare the protective effects of exosomes that are derived from different time periods of breast milk production against intestinal injury using an ex vivo intestinal organoid model. METHODS Colostrum, transitional and mature breast milk samples from healthy lactating mothers were collected. Exosomes were isolated using serial ultracentrifugation and filtration. Exosomes' presence was confirmed using transmission electron microscopy (TEM) and western blot. To form the intestinal organoids, terminal ileum was harvested from neonatal mice pups at postnatal day 9, crypts were isolated and organoids were cultured in matrigel. Organoids were either cultured with exposure to lipopolysaccharide (LPS), or in treatment groups where both LPS and exosomes were added in the culturing medium. Inflammatory markers and organoids viability were evaluated. MAIN RESULTS Human milk-derived exosomes were successfully isolated and characterized. LPS administration reduced the size of intestinal organoids, induced inflammation through increasing TNF$\alpha$ and TLR4 expression, and stimulated intestinal regeneration. Colostrum, transitional and mature human milk-derived exosome treatment all prevented inflammatory injury, while exosomes derived from colostrum were most effective at reducing inflammatory cytokine. CONCLUSIONS Human breast milk-derived exosomes were able to protect intestine organoids against epithelial injury induced by LPS. Colostrum exosomes offer the best protective effect among the breast-milk derived exosomes. Human milk exosomes can be protective against the development of intestinal injury such as that seen in NEC.
SLAS technology 2019
Mutation Profiles in Glioblastoma 3D Oncospheres Modulate Drug Efficacy.
Abstract
Abstract
Glioblastoma (GBM) is a lethal brain cancer with a median survival time of approximately 15 months following treatment. Common in vitro GBM models for drug screening are adherent and do not recapitulate the features of human GBM in vivo. Here we report the genomic characterization of nine patient-derived, spheroid GBM cell lines that recapitulate human GBM characteristics in orthotopic xenograft models. Genomic sequencing revealed that the spheroid lines contain alterations in GBM driver genes such as PTEN, CDKN2A, and NF1. Two spheroid cell lines, JHH-136 and JHH-520, were utilized in a high-throughput drug screen for cell viability using a 1912-member compound library. Drug mechanisms that were cytotoxic in both cell lines were Hsp90 and proteasome inhibitors. JHH-136 was uniquely sensitive to topoisomerase 1 inhibitors, while JHH-520 was uniquely sensitive to Mek inhibitors. Drug combination screening revealed that PI3 kinase inhibitors combined with Mek or proteasome inhibitors were synergistic. However, animal studies to test these drug combinations in vivo revealed that Mek inhibition alone was superior to the combination treatments. These data show that these GBM spheroid lines are amenable to high-throughput drug screening and that this dataset may deliver promising therapeutic leads for future GBM preclinical studies.
Clinical cancer research : an official journal of the American Association for Cancer Research 2019
Mechanisms and Antitumor Activity of a Binary EGFR/DNA-Targeting Strategy Overcomes Resistance of Glioblastoma Stem Cells to Temozolomide.
Abstract
Abstract
PURPOSE Glioblastoma (GBM) is a fatal primary malignant brain tumor. GBM stem cells (GSC) contribute to resistance to the DNA-damaging chemotherapy, temozolomide. The epidermal growth factor receptor (EGFR) displays genomic alterations enabling DNA repair mechanisms in half of GBMs. We aimed to investigate EGFR/DNA combi-targeting in GBM. EXPERIMENTAL DESIGN ZR2002 is a combi-molecule" designed to inflict DNA damage through its chlorethyl moiety and induce irreversible EGFR tyrosine kinase inhibition. We assessed its in vitro efficacy in temozolomide-resistant patient-derived GSCs mesenchymal temozolomide-sensitive and resistant in vivo-derived GSC sublines and U87/EGFR isogenic cell lines stably expressing EGFR/wild-type or variant III (EGFRvIII). We evaluated its antitumor activity in mice harboring orthotopic EGFRvIII or mesenchymal TMZ-resistant GSC tumors. RESULTS ZR2002 induced submicromolar antiproliferative effects and inhibited neurosphere formation of all GSCs with marginal effects on normal human astrocytes. ZR2002 inhibited EGF-induced autophosphorylation of EGFR downstream Erk1/2 phosphorylation increased DNA strand breaks and induced activation of wild-type p53; the latter was required for its cytotoxicity through p53-dependent mechanism. ZR2002 induced similar effects on U87/EGFR cell lines and its oral administration significantly increased survival in an orthotopic EGFRvIII mouse model. ZR2002 improved survival of mice harboring intracranial mesenchymal temozolomide-resistant GSC line decreased EGFR Erk1/2 and AKT phosphorylation and was detected in tumor brain tissue by MALDI imaging mass spectrometry. CONCLUSIONS These findings provide the molecular basis of binary EGFR/DNA targeting and uncover the oral bioavailability blood-brain barrier permeability and antitumor activity of ZR2002 supporting potential evaluation of this first-in-class drug in recurrent GBM."
Oncogene 2019
BMI1 is a therapeutic target in recurrent medulloblastoma.
Abstract
Abstract
Medulloblastoma (MB) is the most frequent malignant pediatric brain tumor, representing 20{\%} of newly diagnosed childhood central nervous system malignancies. Although advances in multimodal therapy yielded a 5-year survivorship of 80{\%}, MB still accounts for the leading cause of childhood cancer mortality. In this work, we describe the epigenetic regulator BMI1 as a novel therapeutic target for the treatment of recurrent human Group 3 MB, a childhood brain tumor for which there is virtually no treatment option beyond palliation. Current clinical trials for recurrent MB patients based on genomic profiles of primary, treatment-naive tumors will provide limited clinical benefit since recurrent metastatic MBs are highly genetically divergent from their primary tumor. Using a small molecule inhibitor against BMI1, PTC-028, we were able to demonstrate complete ablation of self-renewal of MB stem cells in vitro. When administered to mice xenografted with patient tumors, we observed significant reduction in tumor burden in both local and metastatic compartments and subsequent increased survival, without neurotoxicity. Strikingly, serial in vivo re-transplantation assays demonstrated a marked reduction in tumor initiation ability of recurrent MB cells upon re-transplantation of PTC-028-treated cells into secondary recipient mouse brains. As Group 3 MB is often metastatic and uniformly fatal at recurrence, with no current or planned trials of targeted therapy, an efficacious targeted agent would be rapidly transitioned to clinical trials.

 网站首页
网站首页