概要
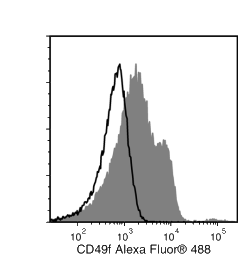
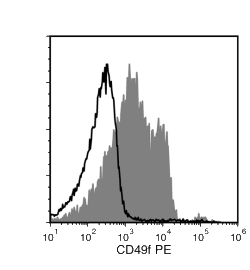
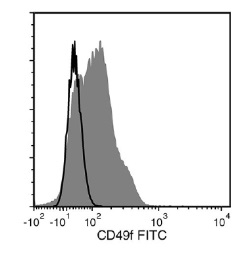
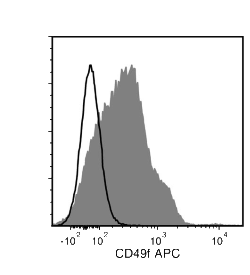
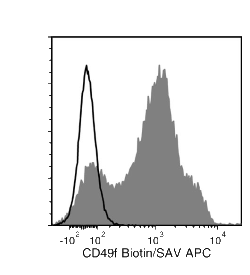
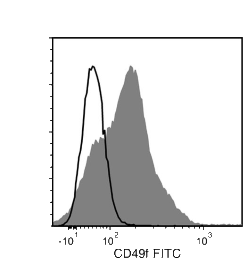
The GoH3 antibody reacts with CD49f (integrin α6), an ~150 kDa transmembrane glycoprotein that associates non-covalently with CD29 (integrin β1) or CD104 (integrin β4) to form the heterodimeric receptors VLA-6 and α6β4, which bind the extracellular matrix protein laminin. CD49f is a disulfide-linked dimer comprising an ~120 kDa heavy chain and an ~30 kDa membrane-bound light chain. Splice variants exist, which affect the cytoplasmic domain of the protein. CD49f is expressed on the surface of T cells, monocytes, platelets, placental trophoblasts, epithelial cells, and endothelial cells. It is involved in cell adhesion and regulating signaling pathways involved in a variety of processes, including the activation and proliferation of T cells, and the differentiation and maintenance of stem cell pluripotency. CD49f is considered the most important marker for selecting mouse mammary stem and progenitor cells. The GoH3 antibody reacts with an extracellular epitope on CD49f and reportedly blocks integrin α6 function in vivo and binding of integrin α6 to laminin in vitro.
技术资料
| Document Type |
产品名称 |
Catalog # |
Lot # |
语言 |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3
|
60037 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Alexa Fluor® 488
|
60037AD, 60037AD.1 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, APC
|
60037AZ, 60037AZ.1 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Biotin
|
60037BT, 60037BT.1 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, FITC
|
60037FI, 60037FI.1 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, PE
|
60037PE, 60037PE.1 |
All |
English |
|
Product Information Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Pacific Blue™
|
60037PB, 60037PB.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3
|
60037 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Alexa Fluor® 488
|
60037AD, 60037AD.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, APC
|
60037AZ, 60037AZ.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Biotin
|
60037BT, 60037BT.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, FITC
|
60037FI, 60037FI.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, PE
|
60037PE, 60037PE.1 |
All |
English |
|
Safety Data Sheet
|
Anti-Mouse CD49f Antibody, Clone GoH3, Pacific Blue™
|
60037PB, 60037PB.1 |
All |
English |
数据及文献
Publications (1)
Stem cells (Dayton, Ohio) 2013
Fibroblast Growth Factor Receptor Signaling Is Essential for Normal Mammary Gland Development and Stem Cell Function
Pond AC et al.
Abstract
Fibroblast growth factor (FGF) signaling plays an important role in embryonic stem cells and adult tissue homeostasis, but the function of FGFs in mammary gland stem cells is less well defined. Both FGFR1 and FGFR2 are expressed in basal and luminal mammary epithelial cells (MECs), suggesting that together they might play a role in mammary gland development and stem cell dynamics. Previous studies have demonstrated that the deletion of FGFR2 resulted only in transient developmental defects in branching morphogenesis. Using a conditional deletion strategy, we investigated the consequences of FGFR1 deletion alone and then the simultaneous deletion of both FGFR1 and FGFR2 in the mammary epithelium. FGFR1 deletion using a keratin 14 promoter-driven Cre-recombinase resulted in an early, yet transient delay in development. However, no reduction in functional outgrowth potential was observed following limiting dilution transplantation analysis. In contrast, a significant reduction in outgrowth potential was observed upon the deletion of both FGFR1 and FGFR2 in MECs using adenovirus-Cre. Additionally, using a fluorescent reporter mouse model to monitor Cre-mediated recombination, we observed a competitive disadvantage following transplantation of both FGFR1/R2-null MECs, most prominently in the basal epithelial cells. This correlated with the complete loss of the mammary stem cell repopulating population in the FGFR1/R2-attenuated epithelium. FGFR1/R2-null MECs were partially rescued in chimeric outgrowths containing wild-type MECs, suggesting the potential importance of paracrine mechanisms involved in the maintenance of the basal epithelial stem cells. These studies document the requirement for functional FGFR signaling in mammary stem cells during development.
View All Publications


 网站首页
网站首页